Application of matrine in preparation of anti-rheumatoid arthritis drugs
A rheumatoid and matrine technology, applied in the field of medicine, can solve problems such as no reports of matrine rheumatoid arthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
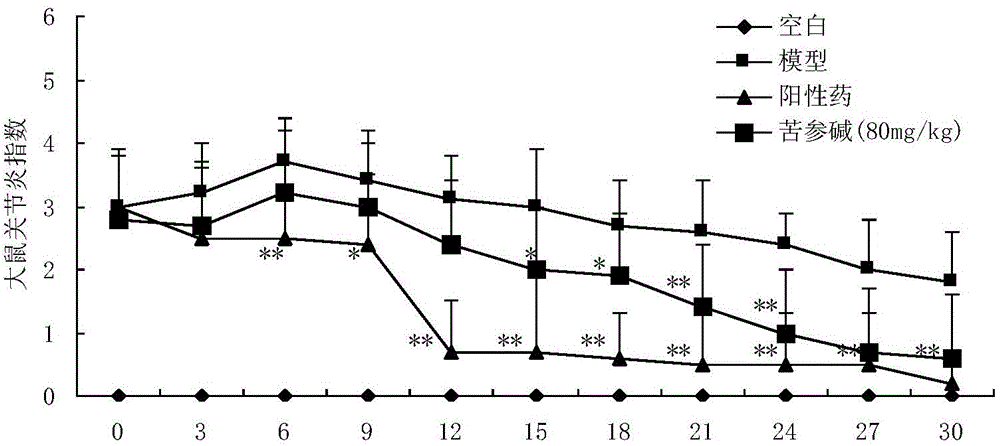
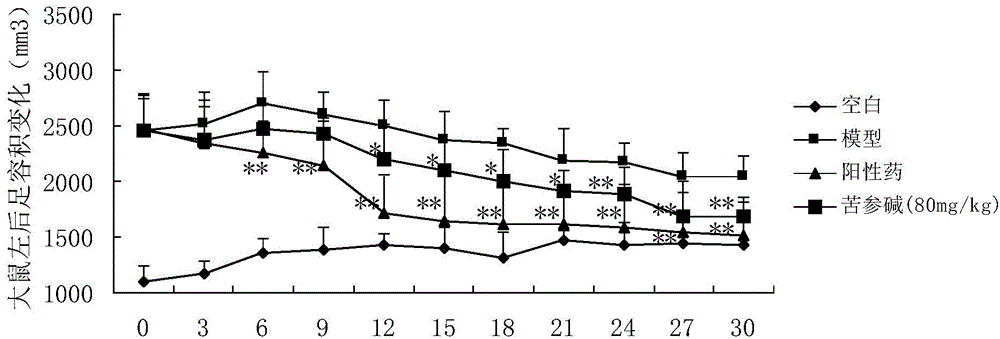
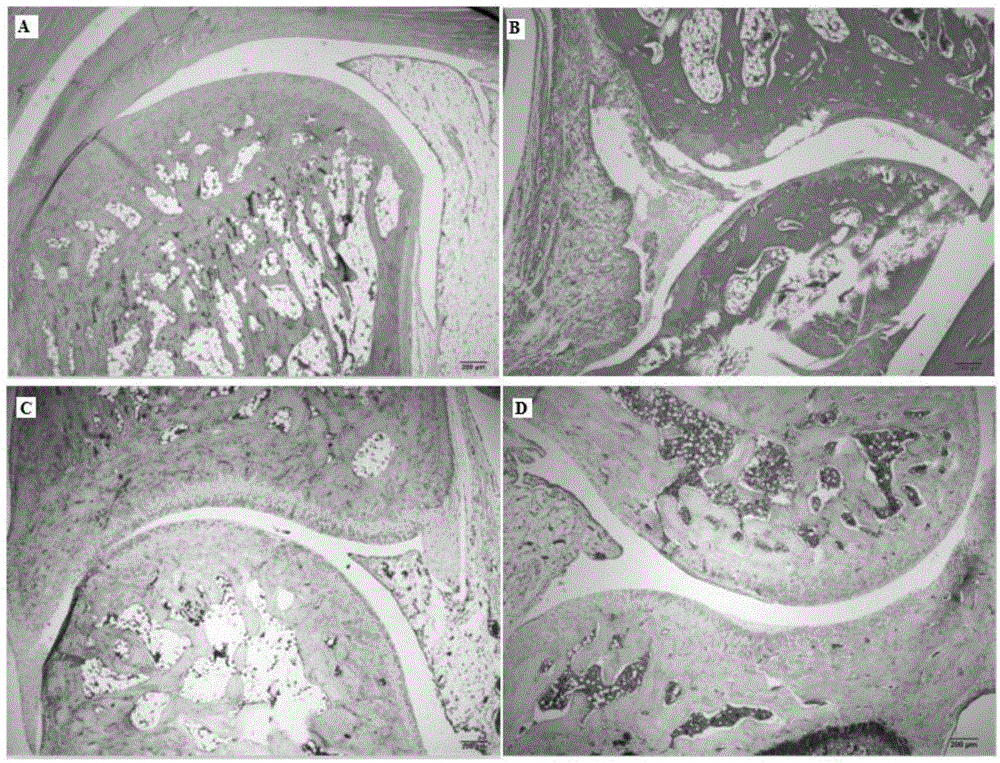
[0021] Example 1: Anti-rheumatoid arthritis pharmacodynamic experiment of matrine
[0022] 1.1 Experimental materials
[0023] 40 adult male SD rats (provided by the Experimental Animal Center of the Second Military Medical University) of 180-220 g, ELISA kits for rat serum TNF-α and IL-1 (Sigma, USA). Main instruments: toe volume measuring instrument (YLS-7A, equipment station of Shandong Academy of Medical Sciences), microplate reader (BIO-RAD 550), centrifuge (Labofuge 400R, Heraeus company), electronic balance (FA 110A type, Shanghai Precision Scientific Instruments Ltd).
[0024] 1.2 Experimental modeling and administration
[0025] Before the experiment, 40 rats were weighed, randomly divided into 4 groups according to body weight, 10 rats in each group, free to eat and drink, and to drink water. Divided into blank group, model group, positive drug group and matrine group. Blank group: without any treatment; model group: bovine type II collagen acetic acid solution w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com