Dehydroepiandrosterone aromatic aldehyde azine steroid compound and its synthesis method and application in the preparation of antitumor drugs
A technology for aromatic aldehyde azine steroids and dehydroepiandrosterone, which is applied in the direction of antineoplastic drugs, steroids, and drug combinations, and can solve problems such as applications that have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
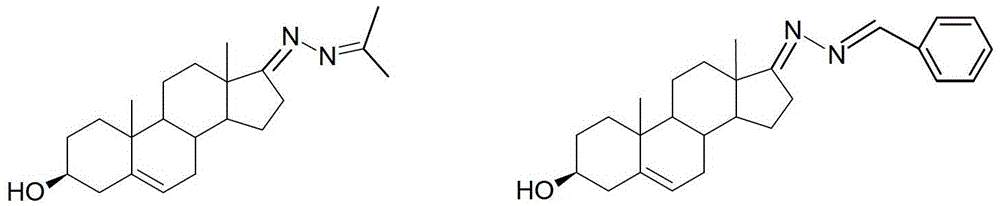
[0024] The preparation of embodiment 1 dehydroepiandrosterone acetone azine (1)
[0025] Step 1: the preparation of dehydroepiandrosterone hydrazone
[0026] Weigh 3.0g of dehydroepiandrosterone and place it in a 100mL round bottom flask, add 40mL of absolute ethanol, stir at room temperature to dissolve completely, then raise the temperature to 45°C, add 4mL of 85% hydrazine hydrate, stir for 2 hours, TLC traces that the raw material point disappears Afterwards, the reaction was terminated, and a white powdery solid was precipitated by distillation under reduced pressure, which was recrystallized from methanol to obtain 2.1 g of a white powder. m.p: 190-192°C. The structure of the product was confirmed by IR, NMR and MS analysis.
[0027] Step 2: the preparation of dehydroepiandrosterone acetone azine (1)
[0028] Weigh 120 mg of dehydroepiandrosterone hydrazone and place it in a 100 mL round bottom flask, add 30 mL of absolute ethanol, stir, add 1 mL of acetone after compl...
Embodiment 2
[0029] The preparation of embodiment 2 dehydroepiandrosterone benzaldehyde azine (2)
[0030] Weigh 60 mg of dehydroepiandrosterone hydrazone, add 25 mL of absolute ethanol, stir at room temperature to dissolve completely, then add 23 mg of benzaldehyde, raise the temperature to 50 degrees, stir for 2 hours, TLC traces that the raw material point basically disappears, terminate the reaction, and depressurize Distill to near dryness, add 15mL water, 15mL ethyl acetate, move into a separatory funnel and extract three times, the organic phase is dried over anhydrous sodium sulfate, and separated by plate chromatography after vacuum distillation. Add petroleum ether with ethyl acetate in a volume ratio of 1: 1.6 was used as the mobile phase to obtain 67 mg of white powder solid with a yield of 86%. m.p: 62-63°C; the structure of the product was confirmed by IR, NMR and MS analyses.
Embodiment 3
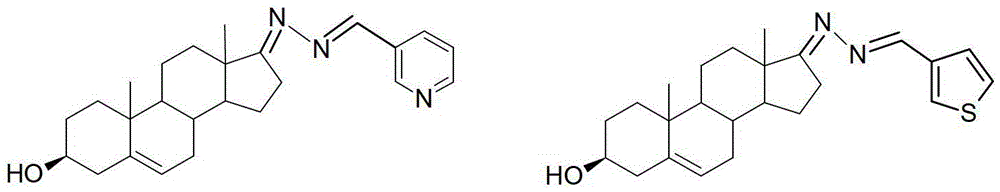
[0031] The preparation of embodiment 3 dehydroepiandrosterone-3-pyridylaldehyde azine (3)
[0032] Weigh 100mg of dehydroepiandrosterone hydrazone, put it in a 100mL round bottom flask, add 25mL of absolute ethanol, add 35mg of 3-pyridinecarbaldehyde after completely dissolving at room temperature, raise the temperature to 40°C and stir for 3 hours, TLC tracking, the raw material point is basically After the disappearance, the reaction was terminated. After distillation under reduced pressure, recrystallization was performed with a mixed solvent of ethyl acetate and petroleum ether to obtain 96 mg of light yellow crystals, with a yield of 70%, m.p: 195-197°C; the structure of the product was determined by IR, NMR and MS analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com