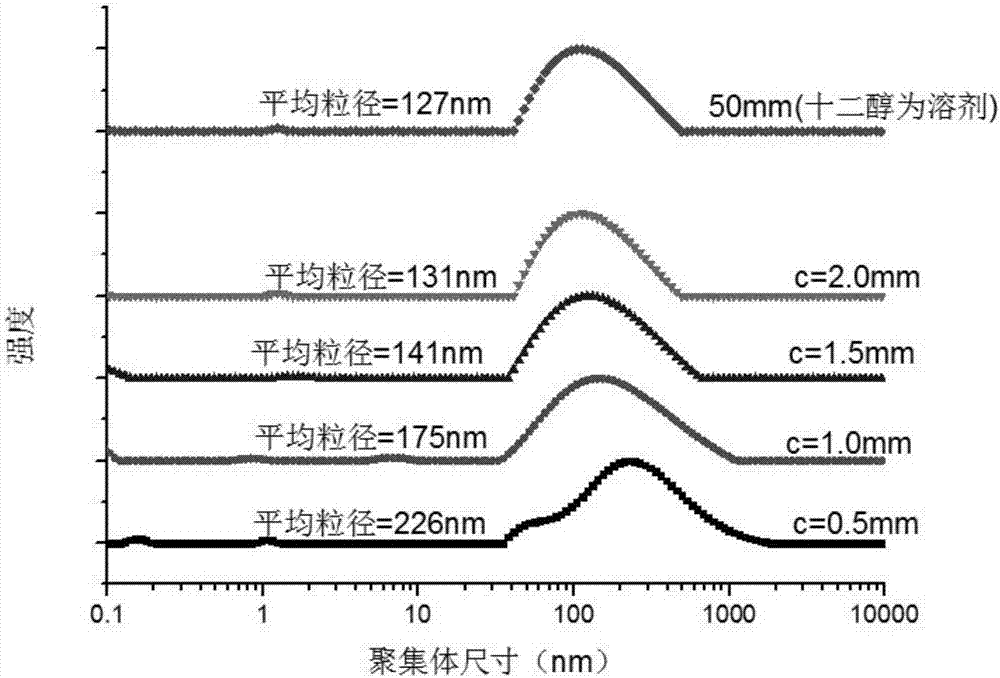
Bio-based primary amine cationic surfactant and preparation method thereof
A technology of surfactant and primary amine cation, applied in the field of bio-based primary amine cationic surfactant and its preparation, can solve the problem of limited research breadth and depth, high synthesis cost, and insufficient types of primary amine cationic surfactants and other problems, to reduce the difficulty, improve the reaction yield, and enhance the water transfer and aggregation capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
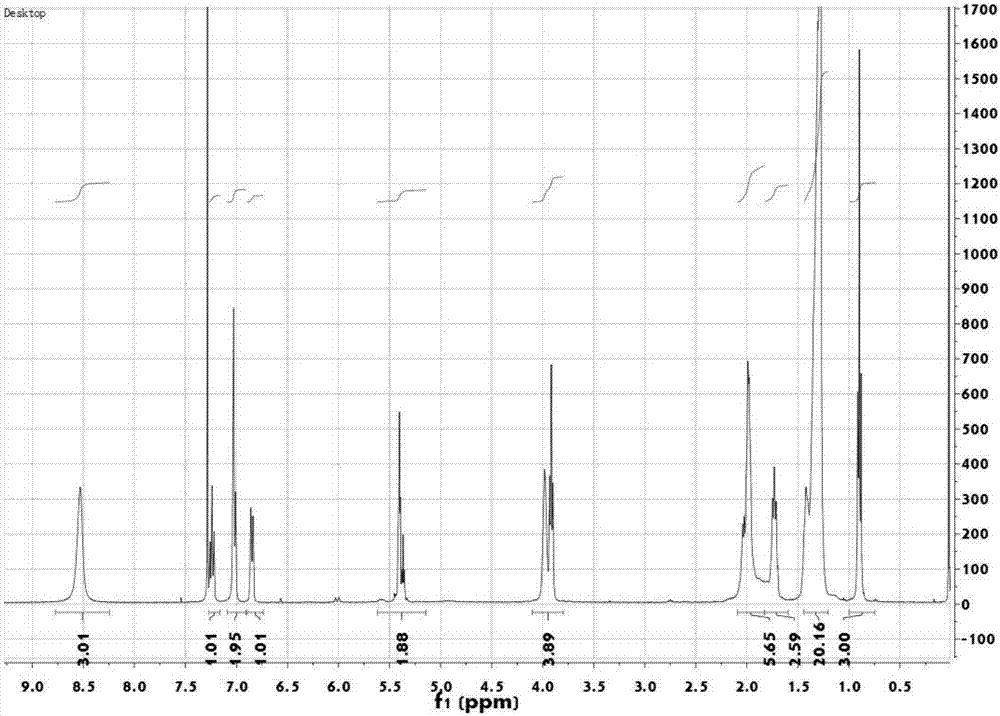
[0048] Embodiment 1: synthetic route of bio-based primary amine cationic surfactant
[0049] Bio-based primary amine cationic surfactant, the structural formula is as follows:
[0050]
[0051] The synthetic route of bio-based primary amine cationic surfactant is as follows:
[0052]
[0053] The preparation method of the novel bio-based primary amine cationic surfactant is characterized in that, the method first takes oleic acid and methanol as the starting raw materials to successively undergo esterification, reduction, and replacement of synthetic oleyl alcohol, oleyl alcohol and 3-hydroxybenzene Formaldehyde undergoes substitution, substitution, reduction, and acidification reactions to obtain the final bio-based primary amine cationic surfactant.
Embodiment 2
[0054] Embodiment 2: the synthesis of methyl oleate
[0055] Centrifuge the centrifuge bottle containing oleic acid at a low temperature of 5°C, and a white solid is precipitated at the bottom. After the solid is filtered off, the liquid can be used for subsequent reactions. Add oleic acid (200g 0.71mol), anhydrous methanol (160g 5mol) and concentrated sulfuric acid (3g) into a 500ml single-necked bottle, and react at 72°C for 4 hours. After the reaction, the methanol is removed by rotary evaporation. Then the organic layer was washed with water, and finally the organic layer was dried with magnesium sulfate, and the pure product of methyl oleate was obtained by suction filtration and vacuum distillation. 172℃~175℃ / 5mmHg. Yield 71%.
Embodiment 3
[0056] Embodiment 3: the synthesis of oleyl alcohol
[0057] Put lithium aluminum hydride (12g, 0.316mol) and 220ml of tetrahydrofuran into a 500ml single-necked bottle, then put it in an ethanol ice bath, then slowly add methyl oleate (90g, 0.3mol) to the reaction with a constant pressure separatory funnel In the system, stir at -10°C for half an hour after the addition, then raise the temperature of the system to 30°C for 3 hours, cool the reactant to -10°C, add 12g of water, 12g of sodium hydroxide dilute solution, 40g of Sodium Sulphate of Water. Mix well and filter, then spin the filtrate to remove the solvent to obtain oleyl alcohol. Yield 80%.
PUM
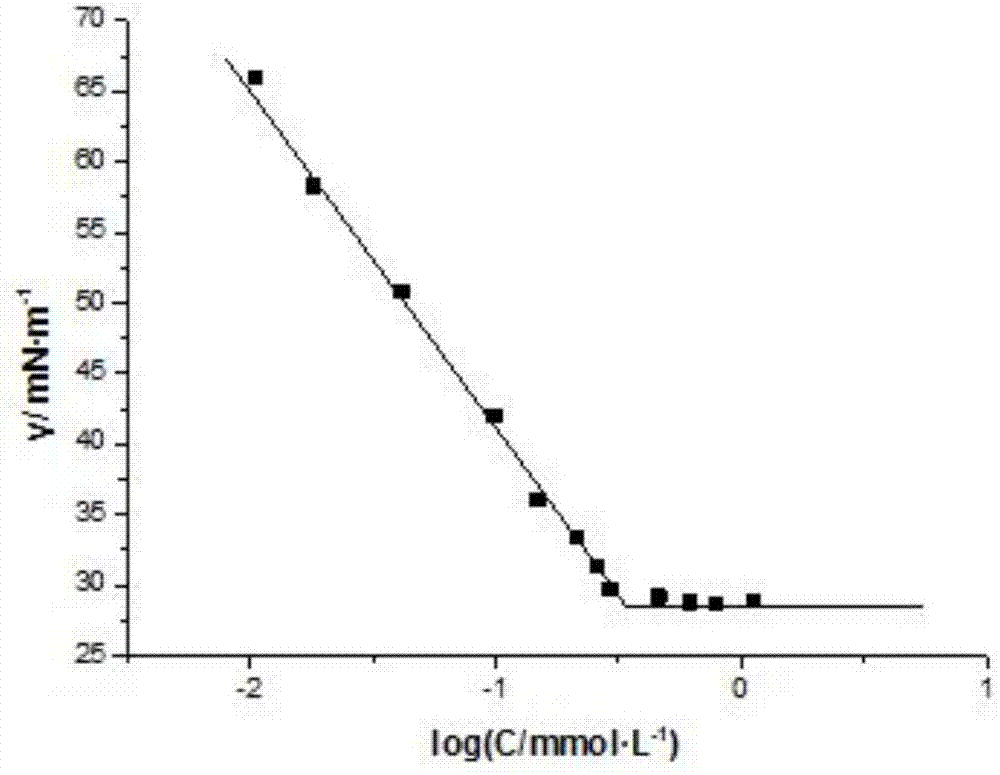
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com