Preparation method of 4-difluoromethoxy-3-hydroxybenzaldehyde
A technology of difluoromethoxybenzaldehyde and hydroxyl group, which is applied in the field of preparation of 3-hydroxy-4-difluoromethoxybenzaldehyde, can solve the problems of low product yield, cumbersome post-processing and high cost, and achieves a product high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
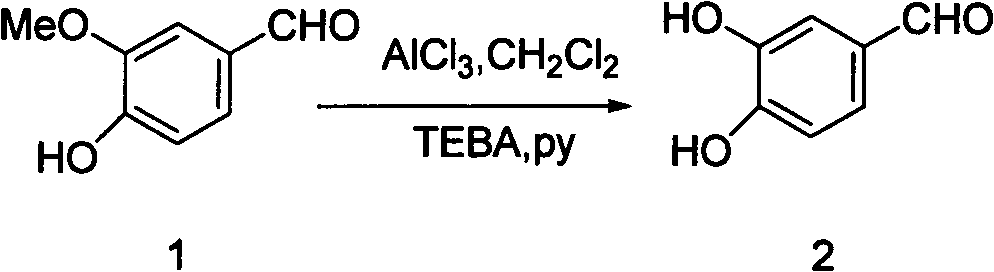
[0016] Embodiment 1: the preparation of 3,4-dihydroxybenzaldehyde
[0017]
[0018] Add vanillin (compound 1) (100.00g) and anhydrous dichloromethane (195ml) into a 1000ml four-neck bottle equipped with a thermometer, a constant pressure dropping funnel and mechanical stirring, stir well at room temperature, add anhydrous trichloromethane Aluminum chloride (123.5 g) and TEBA (triethylbenzyl ammonium chloride) (0.70 g) were stirred at room temperature until the solids were completely dissolved. Slowly and evenly add pyridine (170ml) dropwise at 0-5°C, slowly heat up to reflux (46-48°C), after reflux for 9 hours, the temperature drops below 10°C, adjust pH=2 (1000ml) with 20% hydrochloric acid, The starting material was recovered by extraction with methyl chloride (2×400ml), and the aqueous phase was extracted with ethyl acetate (3×500ml). The combined organic phases were dried over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure, and vacuum-dried...
Embodiment 2
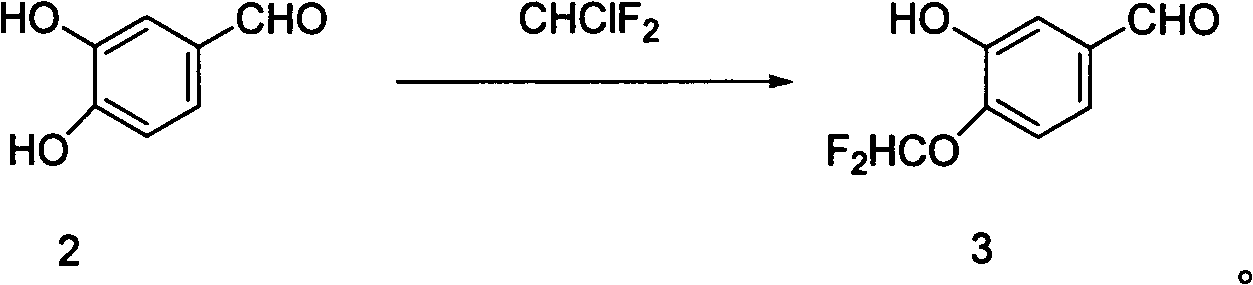
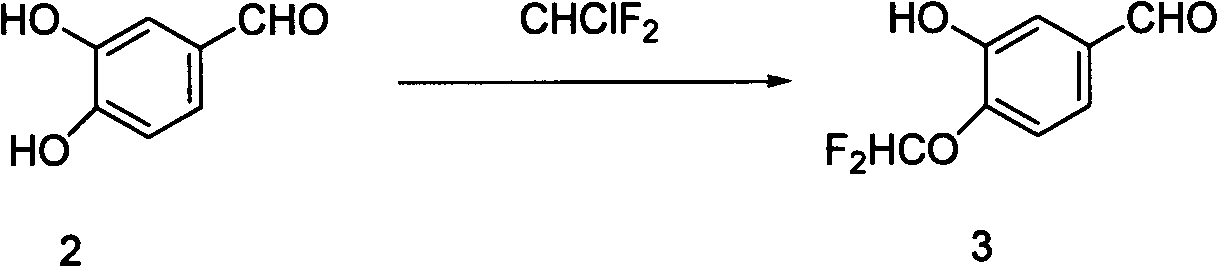
[0019] Embodiment 2: Preparation of 3-hydroxyl-4-difluoromethoxybenzaldehyde
[0020]
[0021] Compound 2 (5.00g, 36.2mmol, 1.0eq) and n-butylammonium bromide (0.117g, 0.362mmol, 1%eq) were dissolved in isopropanol (60ml), stirred at room temperature for 20min, and 30% sodium hydroxide was added Solution (1.59g, 39.82mmol, 1.1eq), stirred at room temperature for 30min, slowly warmed up to 65°C, started to feed chlorodifluoromethane, kept the temperature at 60-65°C, added 30% sodium hydroxide ( 0.43g, 10.86mmol, 0.3eq), continue the reaction, add 30% sodium hydroxide solution (0.43g, 10.86mmol, 0.3eq) after another 1.5h, continue the reaction for 5-6h, drop the temperature to 15°C, add Water terminates the reaction. Filtrate to remove the solid residue, extract the filtrate with ethyl acetate, combine the organic phases, wash with water until neutral, dry over anhydrous sodium sulfate, evaporate the solvent, and perform silica gel column chromatography (petroleum ether: eth...
Embodiment 3
[0022] Embodiment 3: Preparation of 3-hydroxyl-4-difluoromethoxybenzaldehyde
[0023]
[0024] Compound 2 (5.00g, 36.2mmol, 1.0eq) and n-butylammonium bromide (0.117g, 0.362mmol, 1%eq) were dissolved in isopropanol (60ml), stirred at room temperature for 20min, and 30% sodium hydroxide was added Solution (1.59g, 39.82mmol, 1.1eq), stirred at room temperature for 30min, slowly warmed up to 65°C, started to feed chlorodifluoromethane, kept the temperature at 70-75°C, added 30% sodium hydroxide ( 0.43g, 10.86mmol, 0.3eq), continue the reaction, add 30% sodium hydroxide solution (0.43g, 10.86mmol, 0.3eq) after another 1.5h, continue the reaction for 5-6h, the temperature drops to 15°C, add Water terminates the reaction. Filtrate to remove the solid residue, extract the filtrate with ethyl acetate, combine the organic phases, wash with water until neutral, dry over anhydrous sodium sulfate, evaporate the solvent, and perform silica gel column chromatography (petroleum ether: et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com