Fluorescence probe for detecting iron ions, preparation and application
A technology of fluorescent probes and iron ions, which is applied in the field of heavy metal detection, can solve the problems of fewer fluorescent probes, achieve the effects of easy operation, easy-to-obtain products, and avoid the use of organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
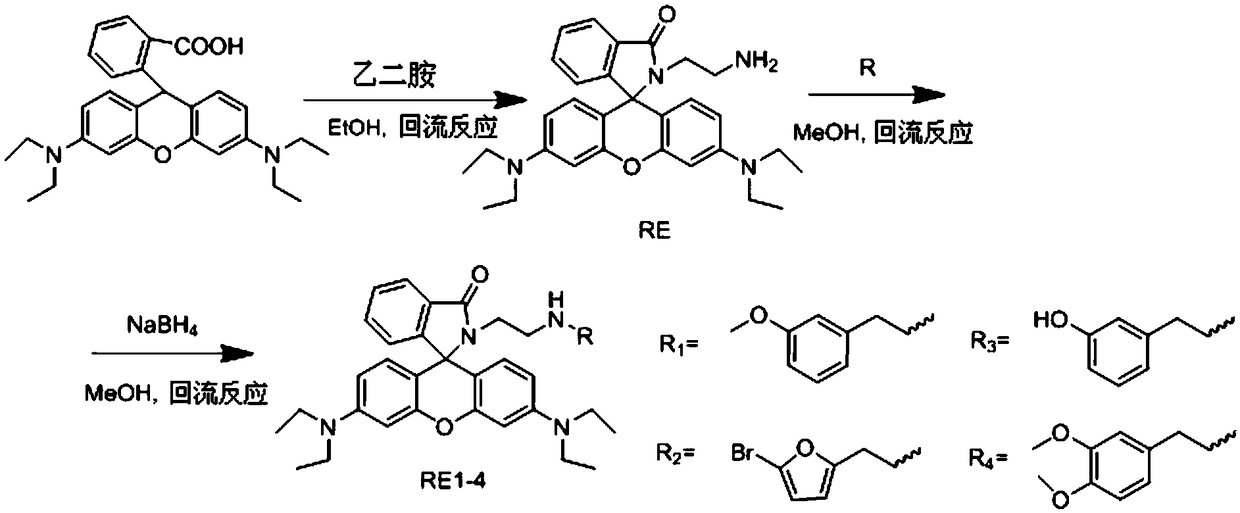
[0041] The synthesis of a kind of fluorescent probe (I) that detects iron ion of the present embodiment, concrete synthetic steps are as follows:
[0042] 1. Synthesis of intermediate RE
[0043] Rhodamine B (4.80g, 10mmol) was added to a 250mL three-neck flask, 100mL of ethanol was added, vigorously stirred at room temperature for 5min, and excess anhydrous ethylenediamine (5mL, 75mmol) was slowly added dropwise, and then the reaction temperature was raised to 80°C React for 4 hours. After the reaction is complete, spin the solvent to dry, add 100 mL of distilled water, extract with dichloromethane (100 mL×3), dry over anhydrous magnesium sulfate, remove the solvent by rotary evaporation, and dry under infrared light for 2 hours to obtain 3.80 g of a pink solid. The yield was 79.0%. The product identification data are as follows:
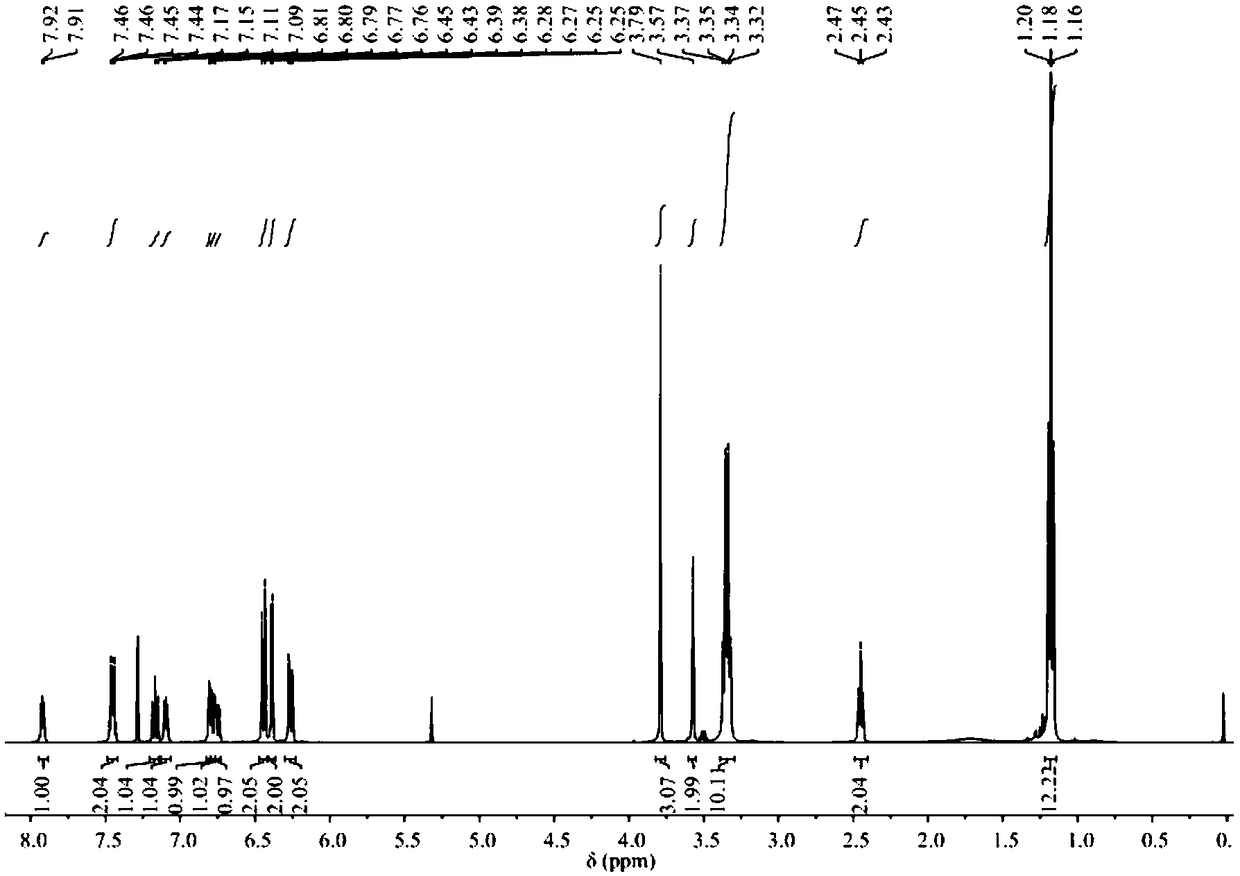
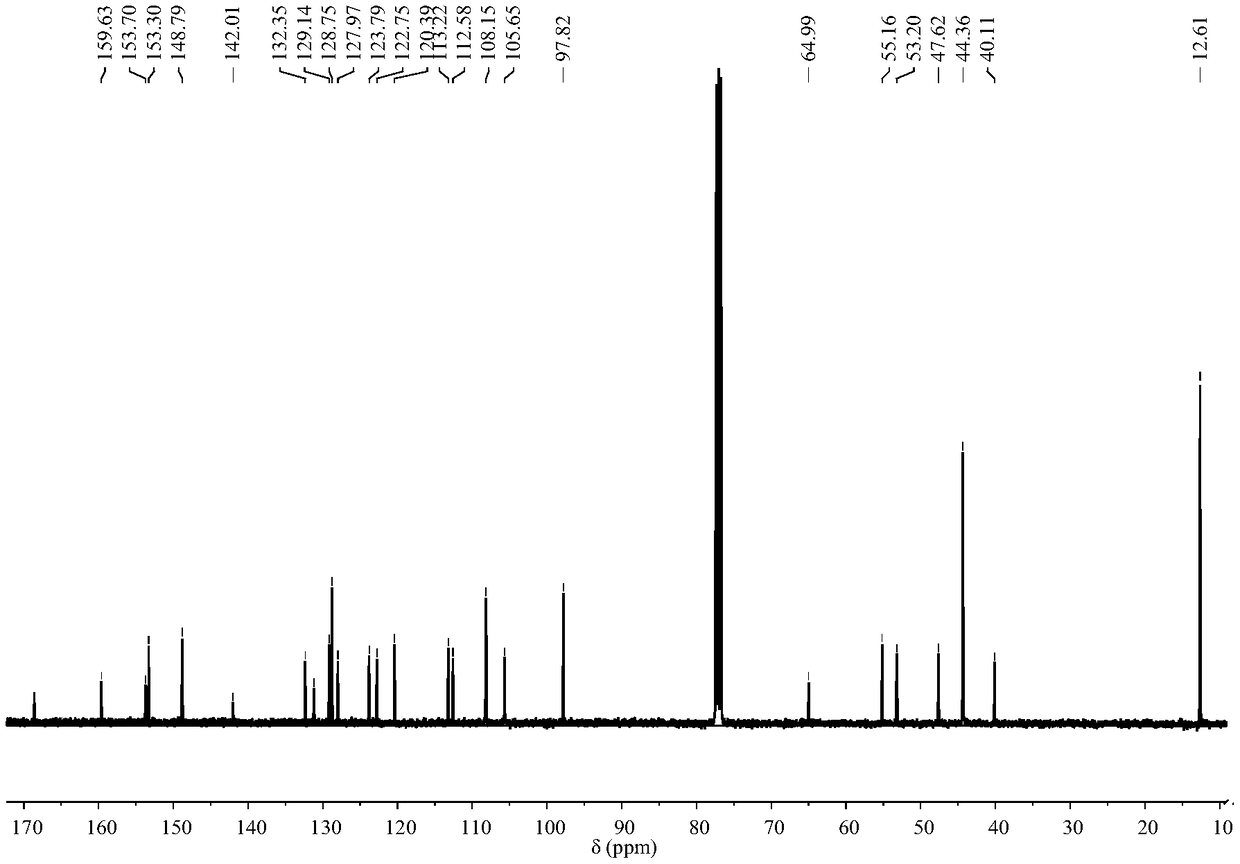
[0044] 1 H NMR (400MHz, CDCl 3 )δ: 7.92(dd, J=5.5,3.0Hz,1H),7.46(dd,J=5.5,3.1Hz,2H),7.11(dd,J=5.3,3.1Hz,1H),6.45(d,J =8.8Hz, 2H), 6.39(d, J=2...
Embodiment 2
[0058] The synthesis of a kind of fluorescent probe (I) that detects iron ion of the present embodiment, concrete synthetic steps are as follows:
[0059] 1. Synthesis of intermediate RE
[0060]Rhodamine B (4.80g, 10mmol) was added to a 250mL three-neck flask, 100mL of ethanol was added, vigorously stirred at room temperature for 5min, and excess anhydrous ethylenediamine (5mL, 75mmol) was slowly added dropwise, and then the reaction temperature was raised to 80°C React for 4 hours. After the reaction is complete, spin the solvent to dry, add 100 mL of distilled water, extract with dichloromethane (100 mL×3), dry over anhydrous magnesium sulfate, remove the solvent by rotary evaporation, and dry under infrared light for 2 hours to obtain 3.75 g of a pink solid. The yield was 77.9%.
[0061] 2. Synthesis of fluorescent probe RE1
[0062] Dissolve 3-methoxybenzaldehyde (0.21g, 1.5mmol) in 20mL of methanol, add dropwise to 30mL of methanol containing RE (0.48g, 1mmol), after t...
Embodiment 3
[0070] The synthesis of a kind of fluorescent probe (I) that detects iron ion of the present embodiment, concrete synthetic steps are as follows:
[0071] 1. Synthesis of intermediate RE
[0072] Rhodamine B (4.80g, 10mmol) was added to a 250mL three-neck flask, 100mL of ethanol was added, vigorously stirred at room temperature for 5min, and excess anhydrous ethylenediamine (5mL, 75mmol) was slowly added dropwise, and then the reaction temperature was raised to 80°C React for 4 hours. After the reaction is complete, spin the solvent to dry, add 100 mL of distilled water, extract with dichloromethane (100 mL×3), dry over anhydrous magnesium sulfate, remove the solvent by rotary evaporation, and dry under infrared light for 2 hours to obtain 3.77 g of a pink solid. The yield was 78.4%.
[0073] 2. Synthesis of small organic molecule probe RE1
[0074] Dissolve 3-methoxybenzaldehyde (0.21g, 1.5mmol) in 20mL of methanol, add dropwise to 30mL of methanol containing RE (0.48g, 1mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com