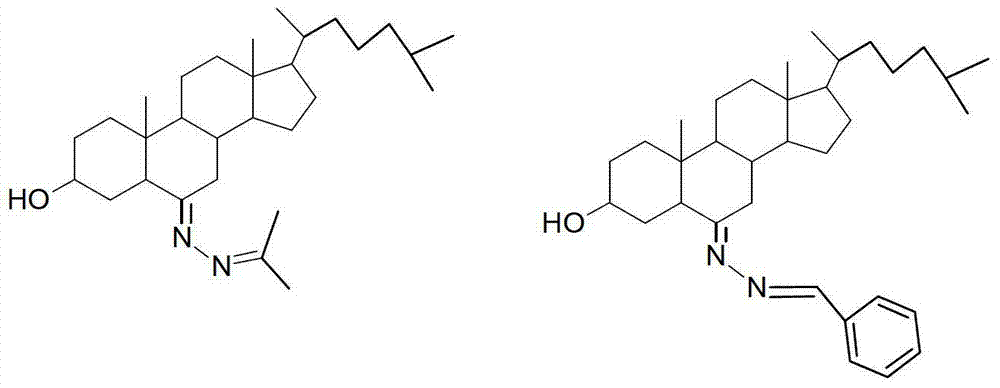
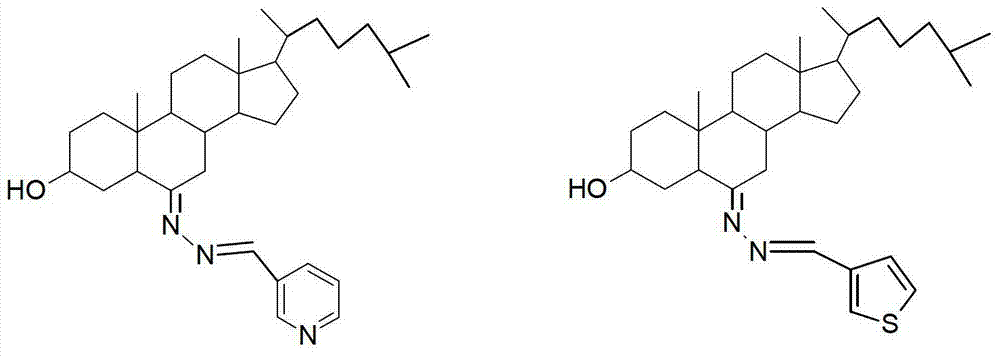
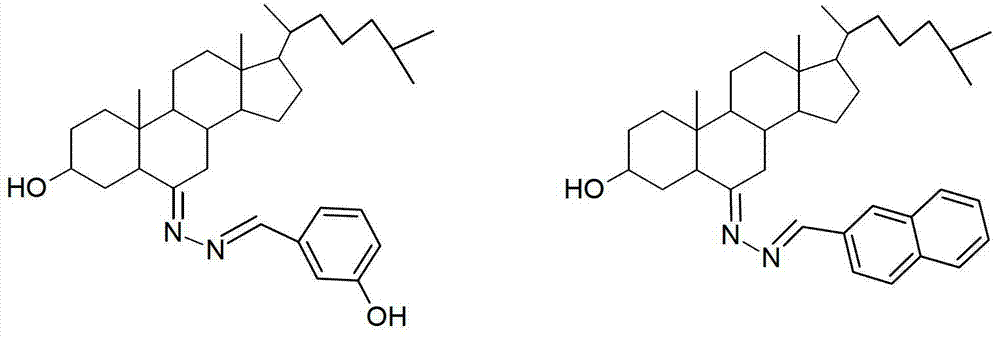
3-hydroxyl cholest-6-keto aromatic aldehyde azine steroidal compound, synthetic method of steroidal compound and application of steroidal compound in preparation of anti-tumour drug
A kind of aromatic aldehyde azine steroid and aromatic aldehyde azine technology, which is applied to 3-hydroxycholest-6-one aromatic aldehyde azine steroid compound and its synthesis and application field in the preparation of antitumor drugs, which can solve the problem of The application has not seen reports and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of 3-hydroxycholesteryl-6-ketoneacetone azine (1)
[0024] Step 1: Preparation of cholest-4-ene-3,6-dione
[0025] Weigh 4g of cholesterol and place it in a 250mL reaction flask, add 100mL of dichloromethane to dissolve the cholesterol completely, then add 10g of PCC, the solution turns black rapidly. Stir the reaction at 35°C for 48h, and stop the reaction after tracking the reaction to the point of no raw material by TLC. The reactant was poured into a short column of silica gel, rinsed with ethyl acetate, the collected solution was evaporated under reduced pressure to remove the solvent, and separated by column chromatography to obtain 3.2 g of a light yellow solid product with a yield of 77%; m.p.131-132°C. The structure of the product was confirmed by IR, NMR and MS analysis.
[0026] Step 2: Preparation of 3β-hydroxycholestan-6-one
[0027] Weigh 200 mg of cholest-4-ene-3,6-dione and 120 mg of nickel chloride hexahydrate, put them in a 100...
Embodiment 2
[0032] Example 2 Preparation of 3-hydroxycholest-6-ketone benzaldehyde azine (2)
[0033] Weigh 60mg of 3-hydroxy-cholester-6-one hydrazone and place it in a 100mL round bottom flask, add 15mL of absolute ethanol to dissolve it completely, add 16mg of benzaldehyde, heat up to 60°C and stir for 3 hours, TLC tracking, raw material point The reaction was terminated after the disappearance, and 55 mg of light yellow crystals were obtained by plate chromatography after vacuum distillation. Yield: 75%, m.p.65-67°C. The structure of the product was confirmed by IR, NMR and MS analysis.
Embodiment 3
[0034] Example 3 Preparation of 3-hydroxycholest-6-one-3-pyridinecarbaldehyde azine (3)
[0035] Weigh 60mg of 3-hydroxy-cholester-6-one hydrazone and place it in a 100mL round bottom flask, add 15mL of absolute ethanol to dissolve it completely, add 17mg of 3-pyridinecarbaldehyde, heat up to 60C° and stir for 3 hours, TLC tracking, raw materials The reaction was terminated after the dots basically disappeared, and 51 mg of brown-red crystals were obtained by plate chromatography after vacuum distillation, yield: 71%, m.p.110-112°C. The structure of the product was confirmed by IR, NMR and MS analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com