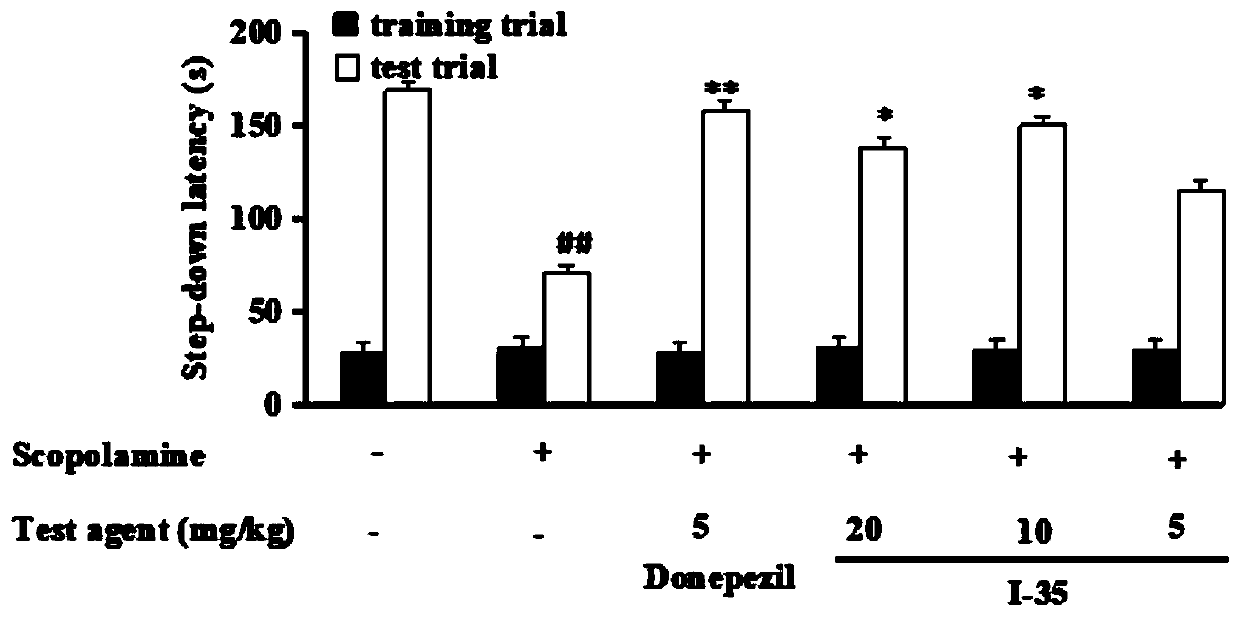
2'-Hydroxyl-3phenyl propiophenone compound and preparation method and application thereof
A technology of phenylpropiophenone and compounds, applied in the field of 2'-hydroxy-3 phenylpropiophenone compounds and their preparation, can solve the problems of poor curative effect, cholinergic side effects, and butyrylcholinesterase inhibitory activity Bad question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
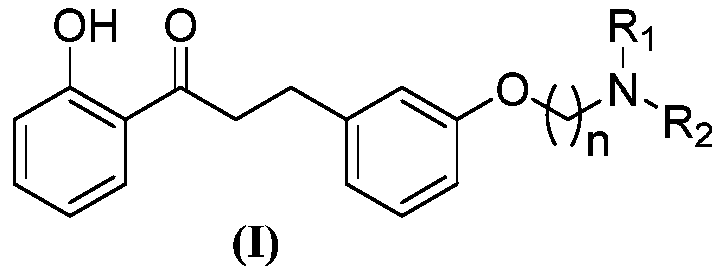
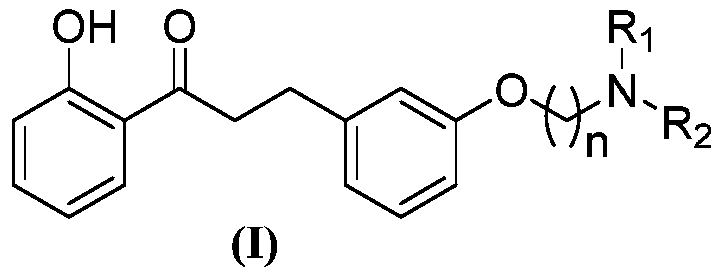
[0043] A kind of 2'-hydroxyl-3 phenyl propiophenone compound, its general chemical structure formula is as shown in (I):
[0044]
[0045] Among them, n, R 1 , R 2 See Table 1 for the definition.
[0046] Table 1 2'-hydroxyl-3 phenylpropiophenone compounds of the present invention
[0047]
[0048]
[0049]
[0050]
[0051] A kind of preparation method of 2'-hydroxyl-3 phenyl propiophenone compound, comprises the following steps:
[0052] (1) Add 2.0mmol of the corresponding 3-hydroxybenzaldehyde (1), 6.0mmol of dibromide (2), 4.0mmol of potassium carbonate and 15mL of acetonitrile into the reaction flask, heat to 65°C for 10 hours, and after the reaction, After conventional treatment, it was purified by silica gel column chromatography (eluent: petroleum ether: acetone = 50:1v / v) to obtain the corresponding alkoxybenzaldehyde (3);
[0053] (2) corresponding alkoxybenzaldehyde (3) 2.0mmol, secondary amine NR 1 R 2 (4) 2.5mmol and 3.0mmol of potassium carb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com