Patents
Literature
170 results about "Propiophenones" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Propiophenone (ethyl phenyl ketone, structural formula C6H5COCH2CH3) and its derivatives. They are commonly used in perfumes and pharmaceuticals.
Ester derivatives of hyaluronic acid for the preparation of hydrogel materials by photocuring
The present invention relates to hyaluronic acid ester derivatives, whose carboxylic groups are partially esterified with hydroxy groups of propiophenone derivatives, to the hydrogel materials consisting of the said hyaluronic acid ester derivatives, to their preparation process by photocuring of the hyaluronic acid ester derivatives, and their use in the biomedical, sanitary and surgical fields, and in the medical field as controlled release systems for drugs.
Owner:FIDIA FARM SPA
Propiophenone derivatives and process for preparing the same
InactiveUS6048842AHigh activityGood hypoglycemic activityAntibacterial agentsBiocideD-GlucopyranoseHydroxy group
A propiophenone derivative of the formula (I): wherein OX is a hydroxy group which may optionally be protected, Y is a lower alkyl group, and Z is a beta -D-glucopyranosyl group wherein one or more hydroxy groups may optionally be protected, or a pharmaceutically acceptable salt thereof. Said compounds have excellent hypoglycemic activity so that they are useful in the prophylaxis or treatment of diabetes.
Owner:MITSUBISHI TANABE PHARMA CORP
Method of preparing R-(+)-3-chlorophenylpropanol
ActiveCN101012147AHigh yieldLow costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTryptophanMethionine biosynthesis
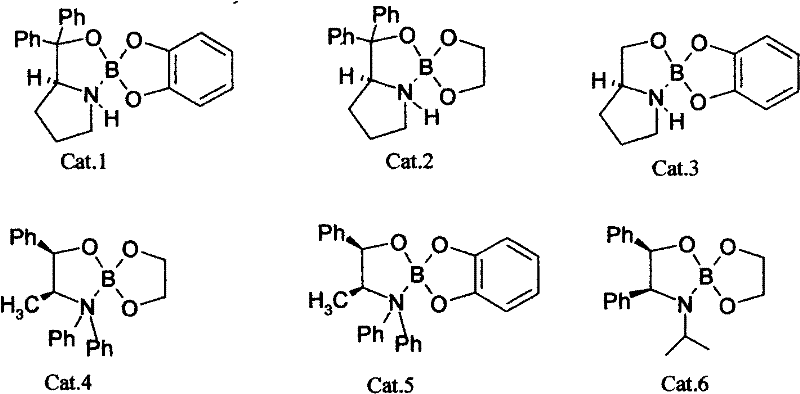
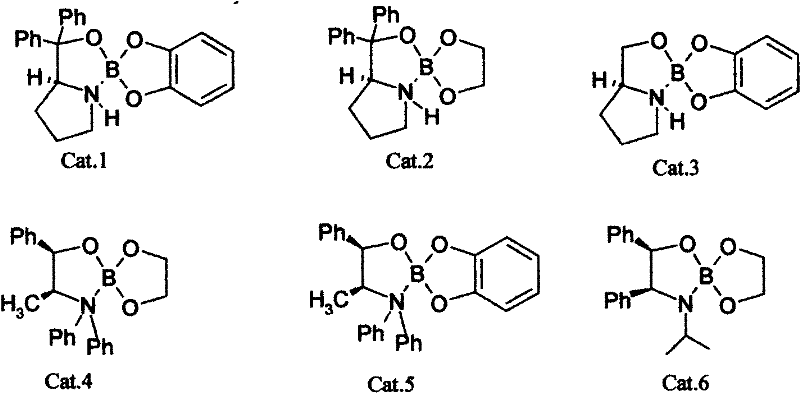
The invention discloses a making method of R-(+)-3-chlophenicol, which is characterized by the following: adopting left-handed bulk of chiral amino acid derivant as catalyst; synthesizing 3-chlophenicol and NaBH4 or KBH4 directly to produce R-(+)-3-chlophenicol; selecting the left-handed bulk from one of left-handed isoleucine derivant, left-handed proline derivant, left-handed tryptophan derivant, left-handed cysteine derivant, left-handed histidine derivant and left-handed methionine derivant.
Owner:XIAMEN FUMAN PHARMA +1
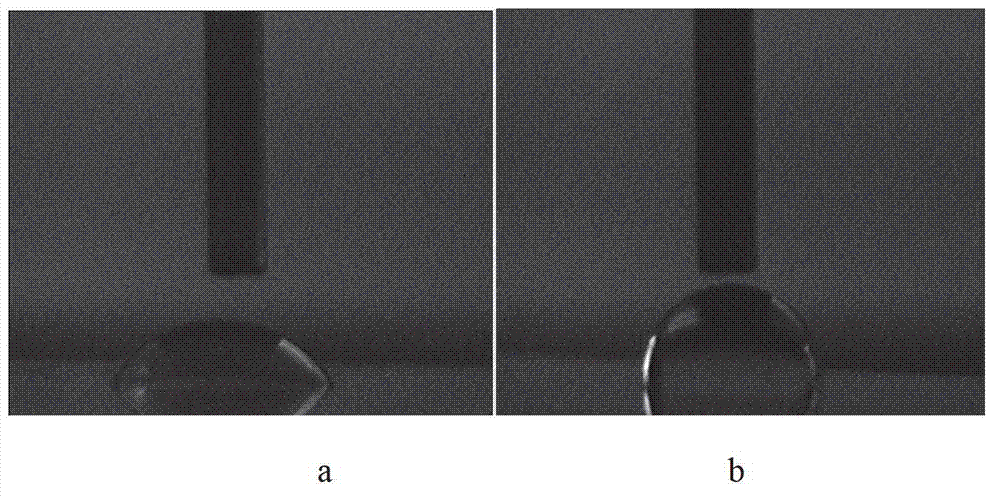
Method for preparing waterproof and antifogging surface modified glass
The invention relates to a method for preparing waterproof and antifogging surface modified glass. The method comprises the following steps of: performing ultraviolet (UV) photo-curing treatment on the modified glass surface by using a photo-curing monomer containing a special functional group to obtain modified waterproof and antifogging glass; preparing silicon-containing photoinitiator is prepared by reacting a silane coupling agent containing isocyanic acid with a photoinitiator 2-hydroxyl-4-(2-hydroxyethoxy)-2-methyl propiophenone (the photoinitiator 2959); and grafting the silicon-containing photoinitiator on the glass surface by hydrolysis reaction to form a chemically-bonded single molecular layer. The product has the characteristics that weather resistance and hydrophobicity of the glass are improved and the binding property between two interfaces is improved by chemical bonding; photo-curing characteristics that the product is environment-friendly, efficient and easy to industrialize are fully utilized; and a way for modification technology development in the future is provided, and organic micromolecules and macromolecules containing special functional groups are grafted on the glass surface.
Owner:BEIJING UNIV OF CHEM TECH
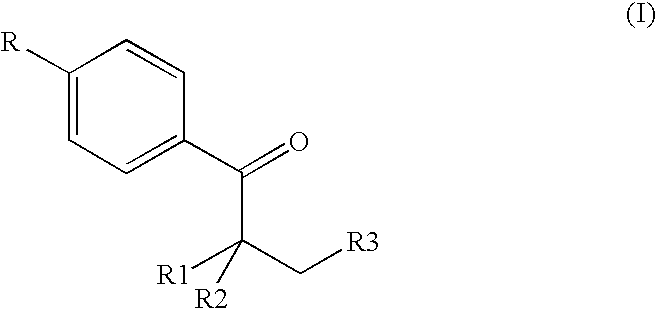
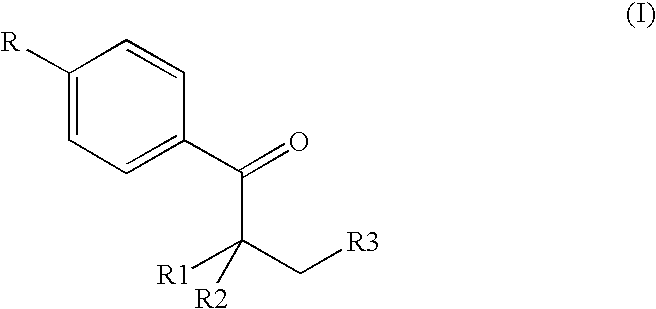
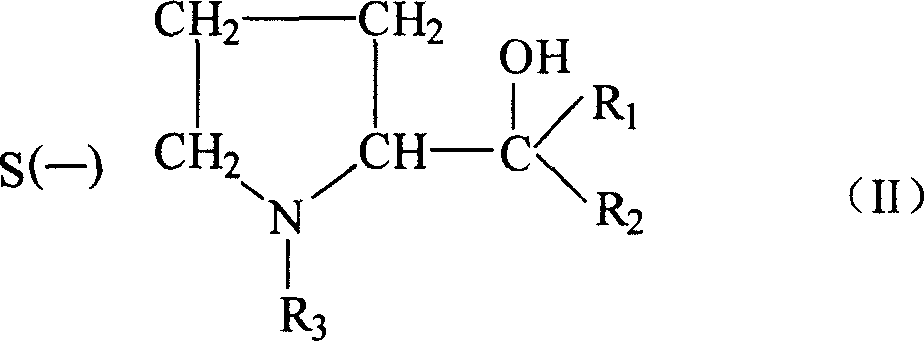
2-amido-2-[2-(4-alkylphenyl)ethyl]-1,3-methyl glycol preparation method
InactiveCN1310869CAtom utilization is highThree wastes lessOrganic compound preparationAmino compound preparationNitroalkaneEthyl group
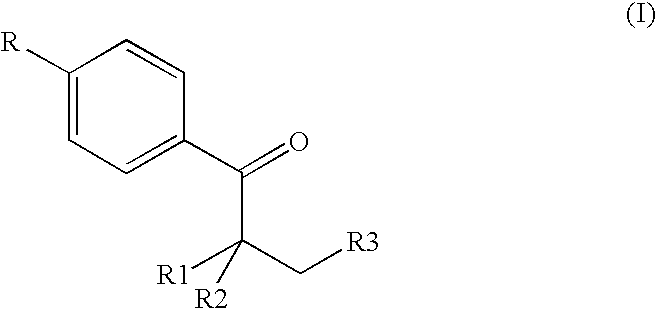
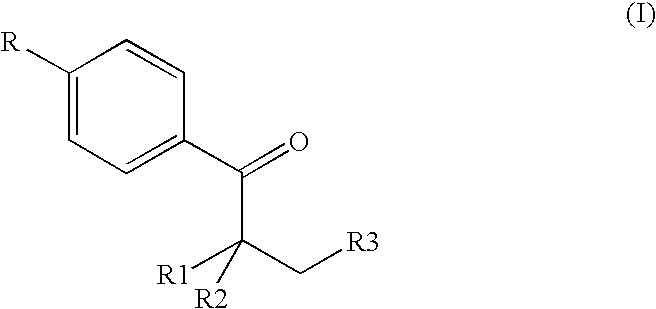
The invention provides a preparation method for (I) 2- amido -2-[2-(4- alkyl phenyl)ethyl]-1,3- propanediol, which comprises: using alkyl benzene (II) as initial material, with Lewis acid existing, to take Friedel-Crafts acylating reaction with 3- halogenated propionyl chloride and generate beta- halogenated alkylpropiophenone (IV); reducing IV by hydride to obtain 3- nitro -1-(4- alkyl phenyl) propanol (V); preparing 2- nitro -2- methylol -4-(4- alkyl phenyl)-1,4butanediol (VI) by hydroxymethylation; reducing nitro and removing benzalcoholhydroxy to VI and obtaining the objective product.
Owner:江苏吴中苏药医药开发有限责任公司 +1
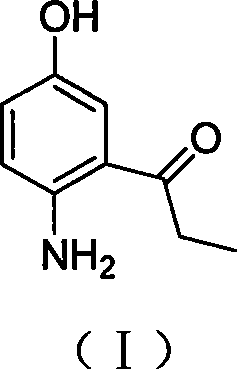
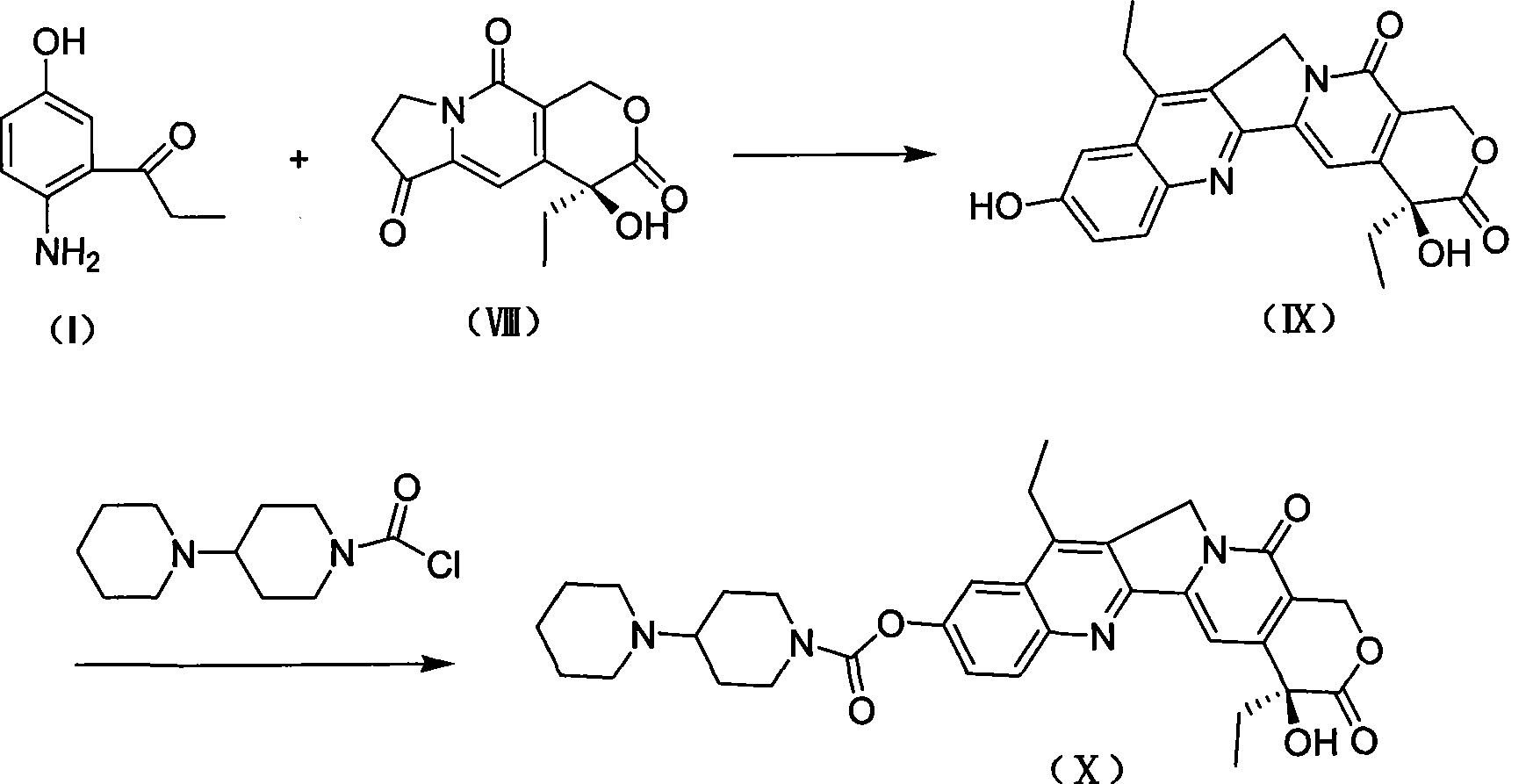
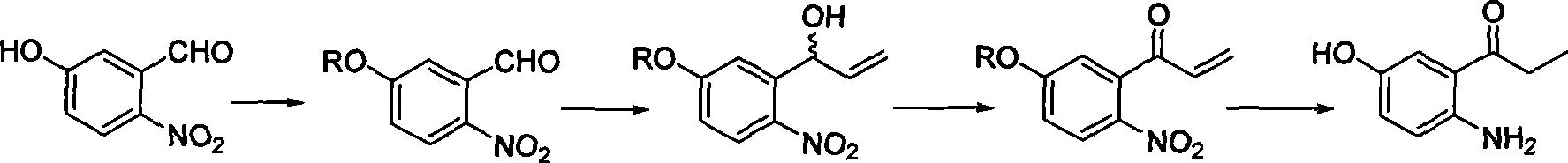
2-amino-5-hydroxypropiophenone preparation method
InactiveCN101362701AHigh yieldLow costOrganic chemistryOrganic compound preparationBenzaldehydePalladium catalyst
The invention relates to a method for preparing an intermediate 2-amino-5-hydroxy propiophenone (I) that is relevant to camptothecin drug synthesizing, which comprises the following steps in sequence: firstly, with the existence of inorganic alkali, 2-nitryl-5-hydroxy benzaldehyde (II) and methylation reagent carry out the O-methylation reaction in solvent to obtain 2-nitryl-5-methoxy benzaldehyde (III); secondly, the compound (III) and hydroxylamine hydrochloride react by a two-step method or a one-step method to obtain 2-nitryl-5-methoxy cyanobenzene (V); thirdly, with the catalysis of the palladium catalyst, the compound (V) carries out the hydrogenation reaction in organic solvent to obtain 2-amino-5-methoxy cyanobenzene (VI); fourthly, under the protection of inert gases, the compound (VI) and format reaction reagent carry out the Grignard reaction in the organic solvent to obtain 2-amino-5-methoxy propiophenone (VII); and fifthly, the compound (VII) and the demethylating reagent carry out the demethylating reaction to obtain 2-amino-5-hydroxy propiophenone (I).
Owner:FUDAN UNIV
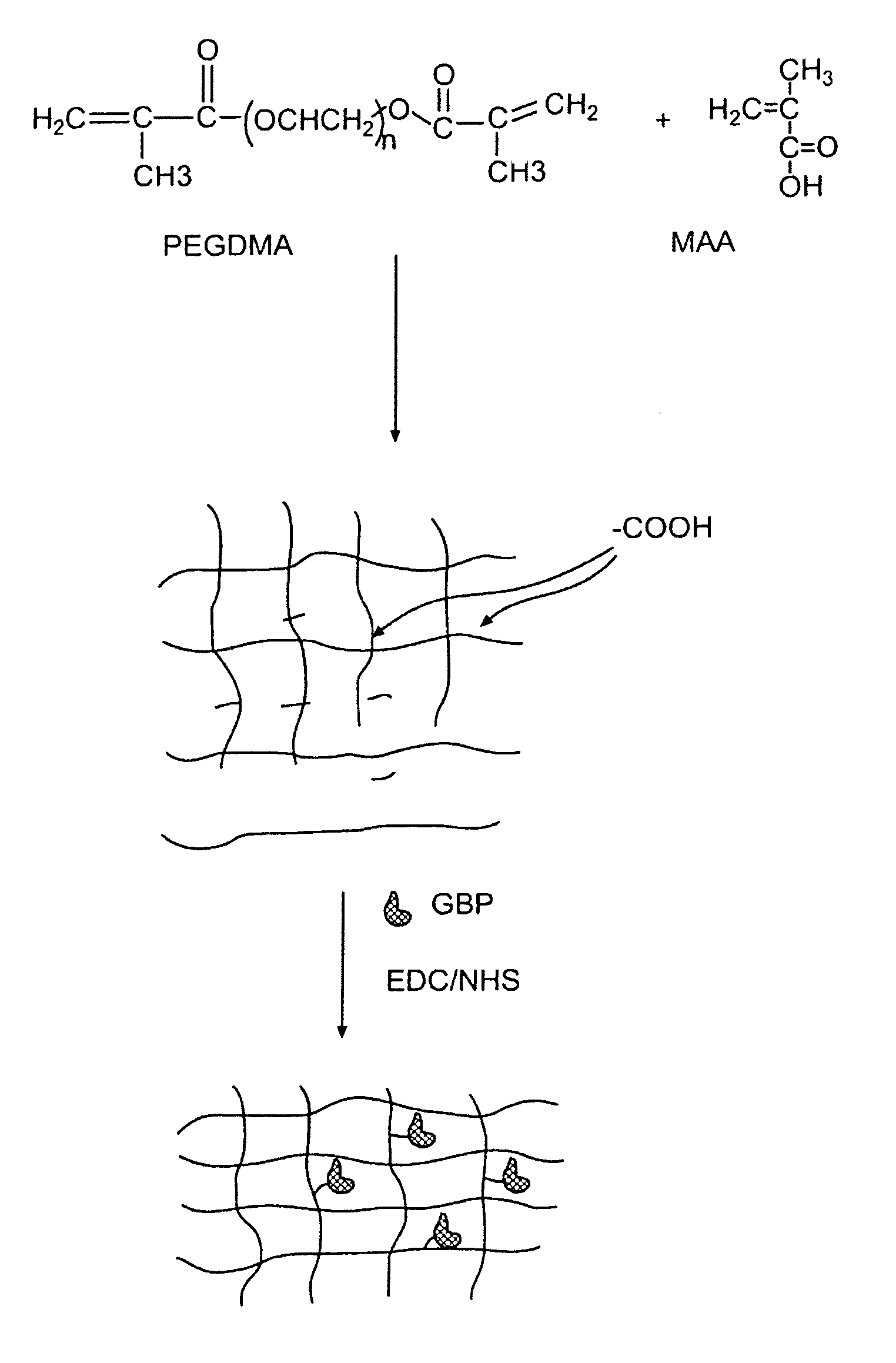
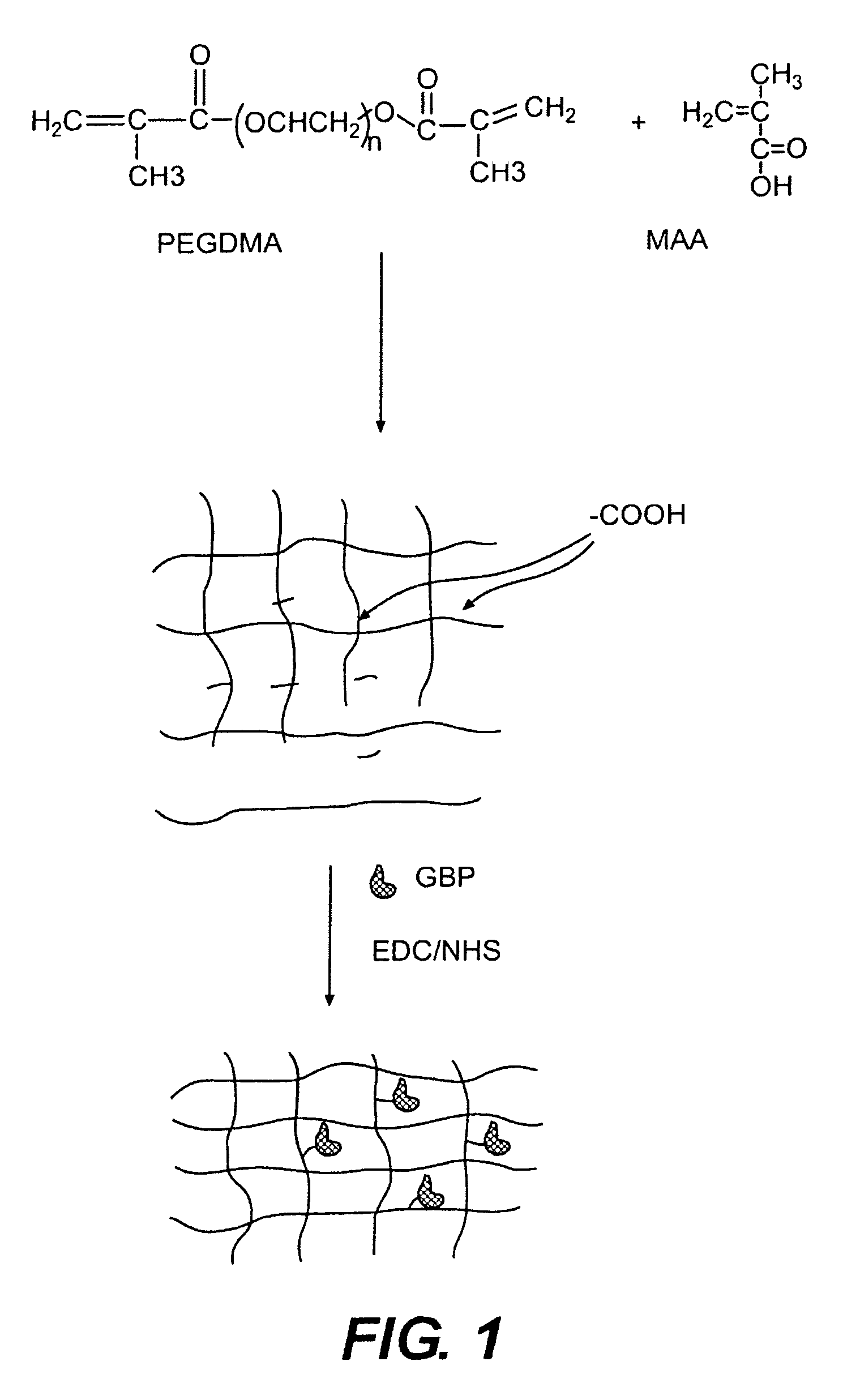
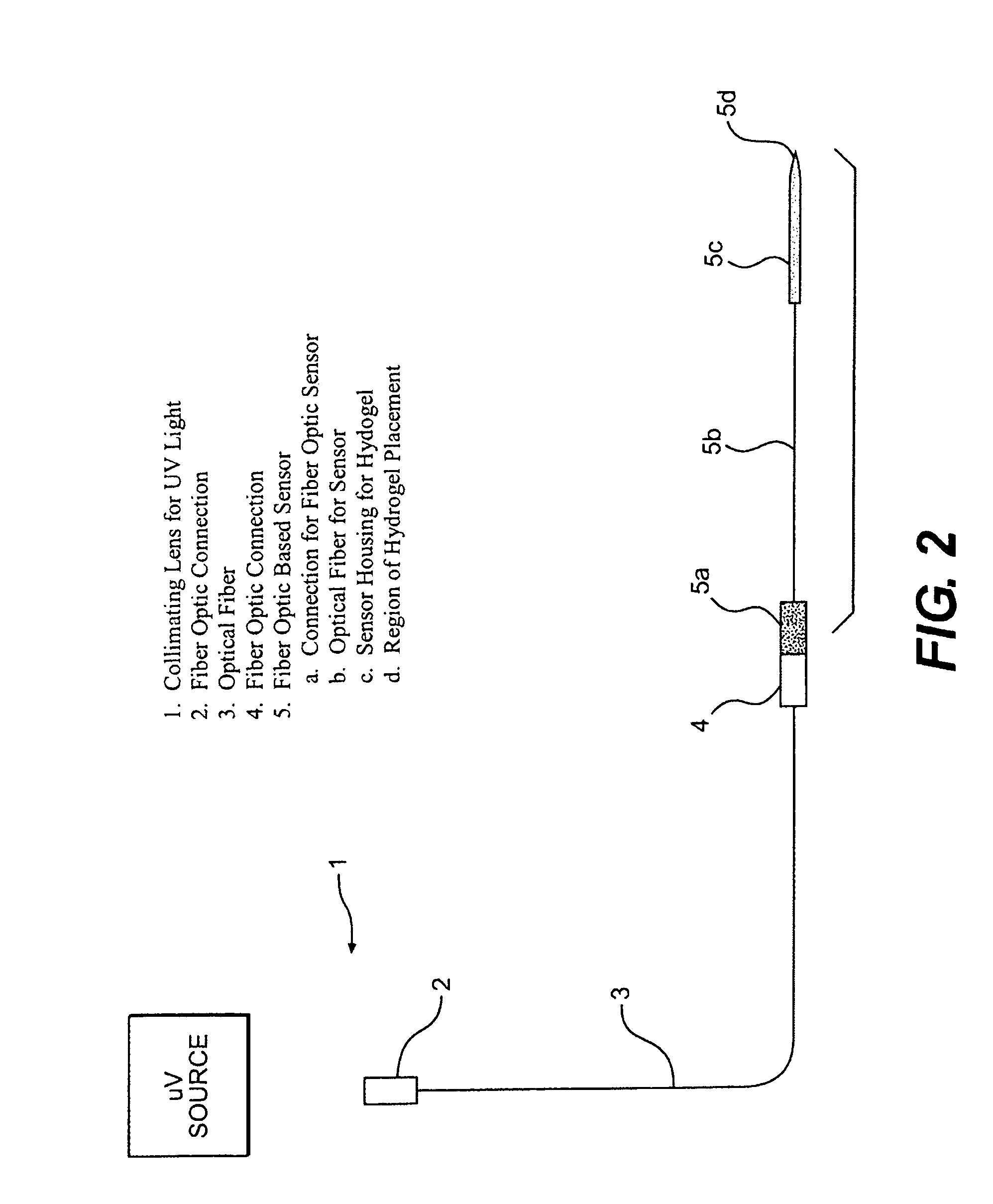
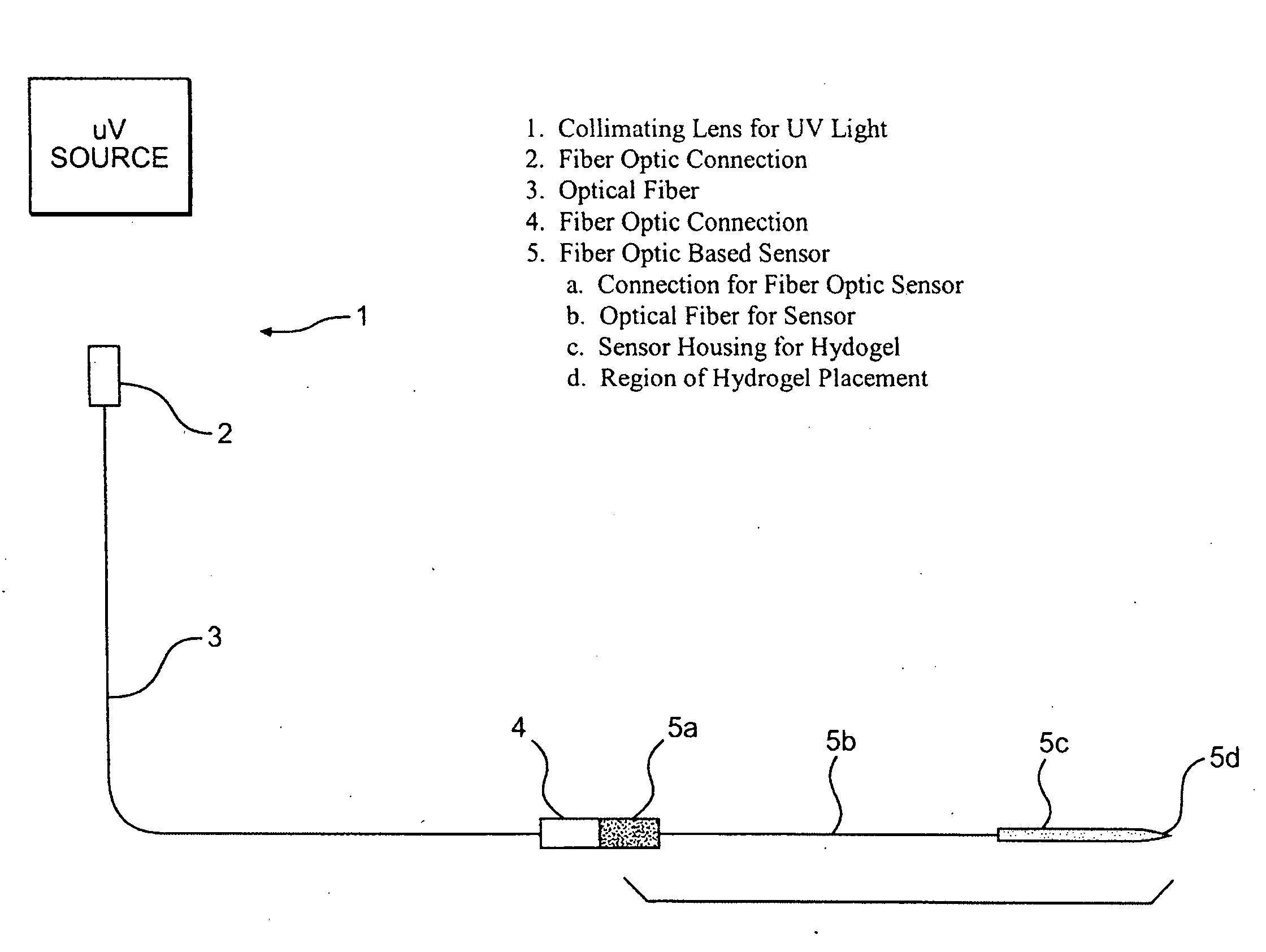
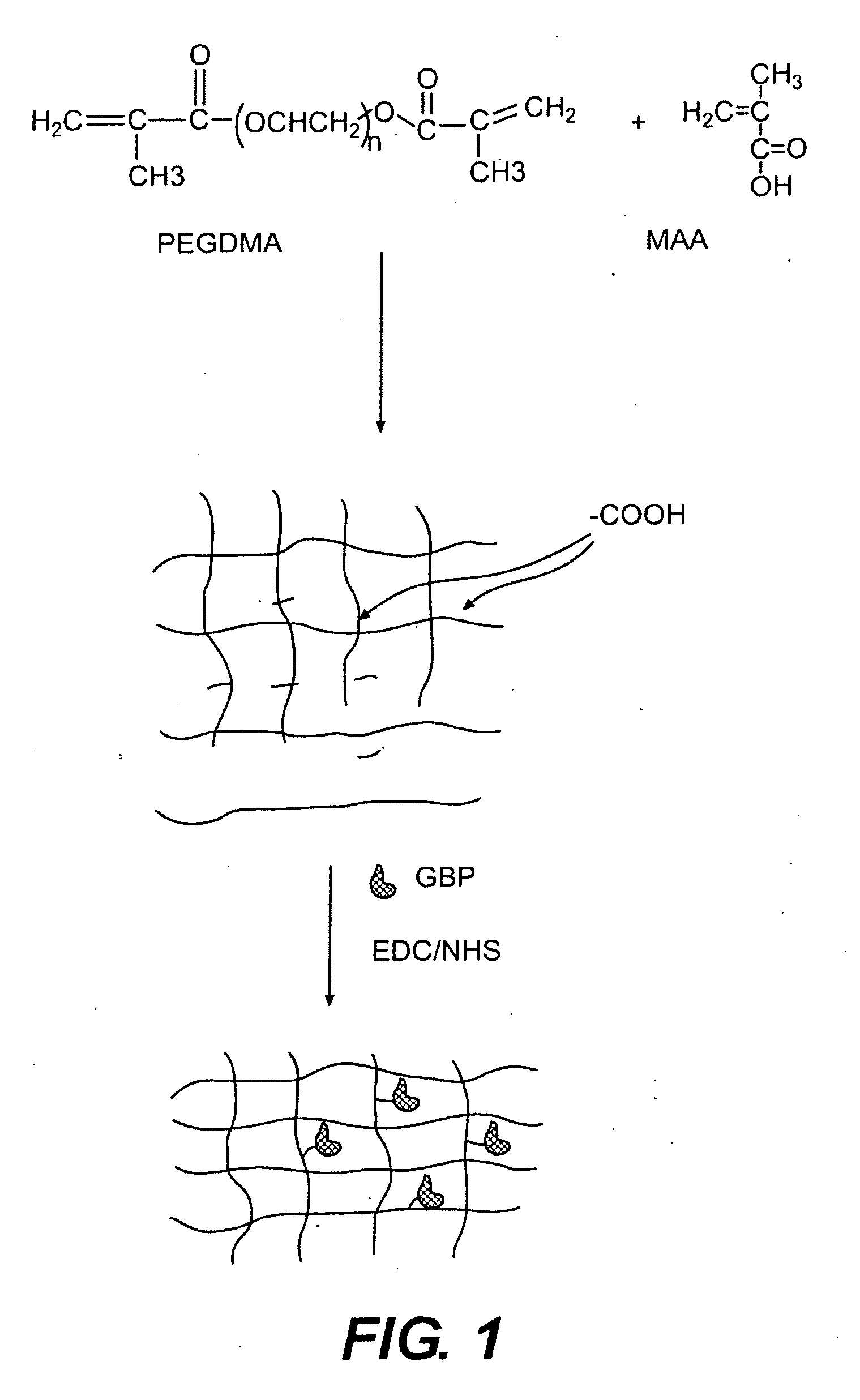
Hydrogel compositions
The invention relates to compositions comprising a hydrogel matrix, where the matrix comprises poly(ethylene glycol) dimethyacrylate (PEGDMA), an acrylate, such as methacrylic acid (MAA) and methyl methacrylate (MMA), as well as 2-hydroxy-2 methyl propiophenone (HMPP).
Owner:BECTON DICKINSON & CO
Ester derivatives of hyaluronic acid for the preparation of hydrogel materials by photocuring
The present invention relates to hyaluronic acid ester derivatives, whose carboxylic groups are partially esterified with hydroxyl groups of propiophenone derivatives, to the hydrogel materials consisting of the said hyaluronic acid ester derivatives, to their preparation process by photocuring of the hyaluronic acid ester derivatives, and their use in the biomedical, sanitary and surgical fields, and in the medical field as controlled release systems for drugs.
Owner:FIDIA FARM SPA
Method for preparing polymerizable photoinitiators
InactiveCN105859551AImprove migration abilityLow mobilityOrganic compound preparationCarboxylic acid esters preparationHydroxybenzoate EthersAdhesive
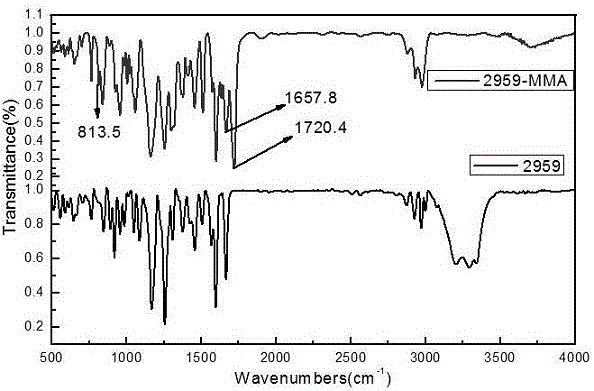
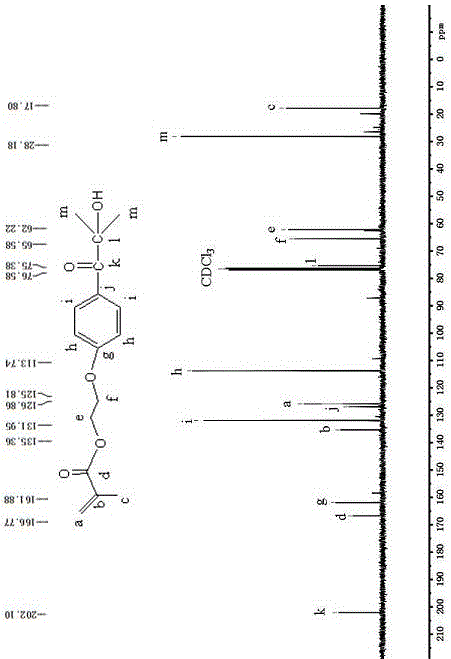
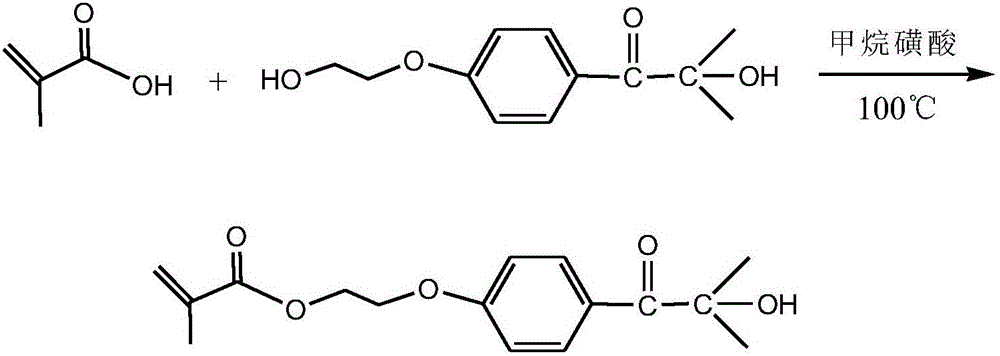
The invention discloses a method for preparing polymerizable photoinitiators. The polymerizable photoinitiators are prepared from methacrylic acid and 2-hydroxyl-4'-(2'-hydroxyethoxy)-2-methyl propiophenone by the aid of direct esterification processes. The use quantity of polymerization inhibitors in a reaction system accounts for 0.01%-0.5% of the mass of the methacrylic acid, the use quantity of methane sulfonic acid which is a catalyst accounts for 0.1%-2% of the mass of the methacrylic acid, and a molar proportion of the methacrylic acid to the 2-hydroxyl-4'-(2'-hydroxyethoxy)-2-methyl propiophenone is 1:1-1.2. Each polymerization inhibitor is methoxyphenol or hydroquinone or 2-tertiary butylhydroquinone, and a solvent is toluene or dimethylformamide or tetrahydrofuran. The method has the advantages that the polymerizable photoinitiators are excellent in compatibility with monomers and resin in photo-curing systems fragments obtained after the polymerizable photoinitiators are subjected to illumination pyrolysis are low in migration rate in cured films and are anti-yellowing, excellent initiation effects can be realized, and the method can be applied to the field of photo-cured coating, printing ink, adhesive and the like; the method for synthesizing the polymerizable photoinitiators is low in cost, processes for preparing the polymerizable photoinitiators are simple and convenient, and obvious application effects can be realized.
Owner:NANCHANG HANGKONG UNIVERSITY
Antifreeze polysaccharide composite starch hydrogel, preparation method and application
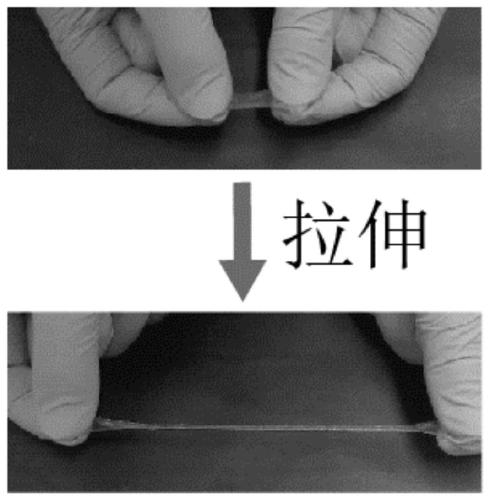
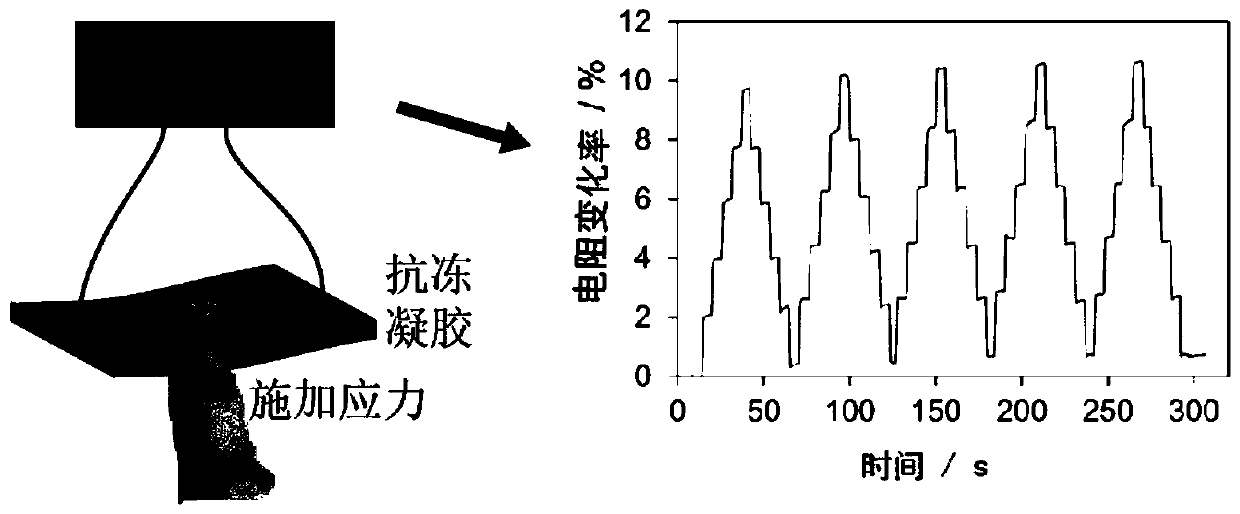
The invention discloses an antifreeze polysaccharide composite starch hydrogel, a preparation method and application. Antifreeze polysaccharide and commercial starch are adopted as the matrix, N-isopropylacrylamide (NIPAM) is used as the cross-linking monomer, N' N'-methylene bisacrylamide (MBA) is employed as the cross-linking agent, 2-hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone is taken as the initiator, and photochemical cross-linking is carried out to prepare the hydrogel with tensile properties, and the elongation of the hydrogel reaches 600%. The hydrogel has antifreeze performance, can maintain good flexibility at minus 70DEG C, has strong response to stress stimulation, high reaction sensitivity (0-1 s) and conductivity, and can output stress as an electrical signal, has stretchable resilience, can be applied as a material for preparation of bionic skin and wearable devices in freezing cold environments.
Owner:EAST CHINA NORMAL UNIVERSITY +1
Environment-friendly binder
InactiveCN105400452AIngredients are easy to getSimple manufacturing methodNon-macromolecular adhesive additivesPolyureas/polyurethane adhesivesEthylic acidPropiophenones
The invention discloses an environment-friendly binder, and a preparing method and application thereof. The environment-friendly binder does not contain phenol, formaldehyde, cresol, acetaldehyde, hyacinthin, methylbenzene, ethylbenzene, acetone, diisocyanate, vinyl acetate and epichlorohydrin and other non-environment friendly organic solvents. Raw materials in a formula are easy to obtain, the preparing method is easy and feasible, and the environment-friendly binder can be used for binding internal wall tiles, wallpaper, wall cloth, ceramic, carpet, office supplies and the like and has good binding effects.
Owner:郭敏
Preparation method of rosuvastatin
ActiveCN102311457AReduce pollutionFew reaction stepsGroup 5/15 element organic compoundsAcyl groupPhenyl group
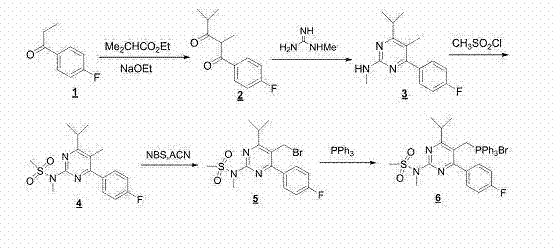
The invention discloses a preparation method of rosuvastatin, comprising the following steps of: using 4-fluoropropiophenone as a raw material, followed by a condensation reaction with ethyl isobutyrate to obtain 1-(4-fluorophenyl)-2,4-dimethylpentane-1,3-dione, performing a cyclization reaction with 1-methylguanidine to obtain 4-(4-fluorophenyl)-6-isopropyl-N,5-dimethyl pyrimidine-2-amine, followed by mesyl substituent and bromination to obtain N-(5-(bromomethyl)-4-(4-fluorophenyl)-6-isopropyl pyrimidine-2-yl)-N-methyl methanesulfonamide, and reacting with triphenyl phosphine to form a wittig reagent so as to prepare rosuvastatin provided by the invention. By the adoption of the preparation method provided by the invention, reaction steps are shortened from nine step reactions in original technology to fine step reactions; in addition, two oxidation reactions and an ultralow temperature reduction reaction are avoided, the production efficiency is effectively increased, the product quality is raised, the environmental pollution is minimized, and the production cost is reduced.
Owner:苏州莱克施德药业有限公司
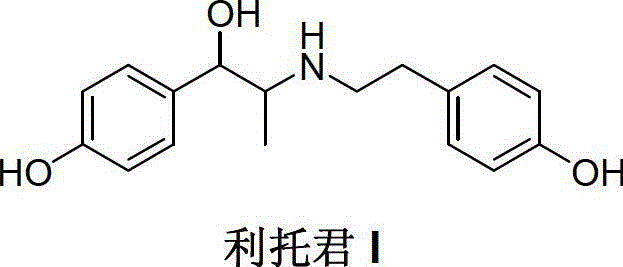
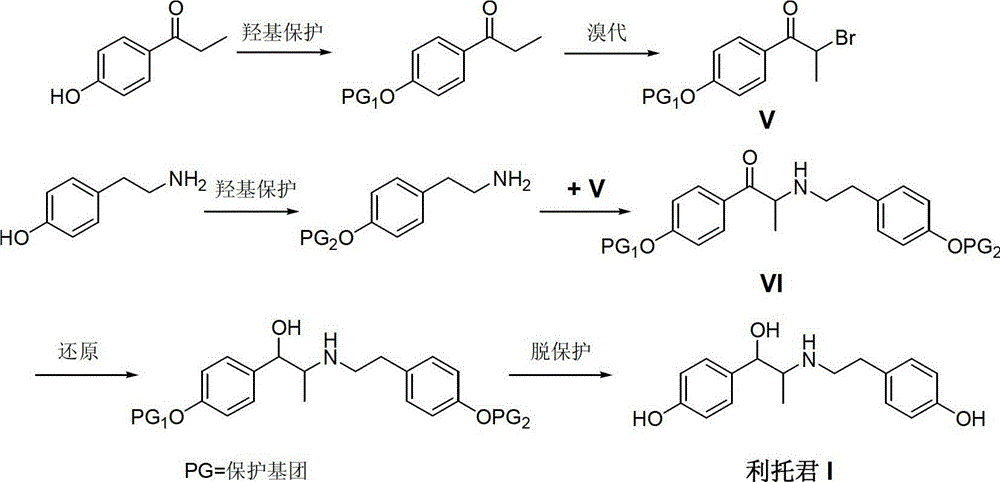
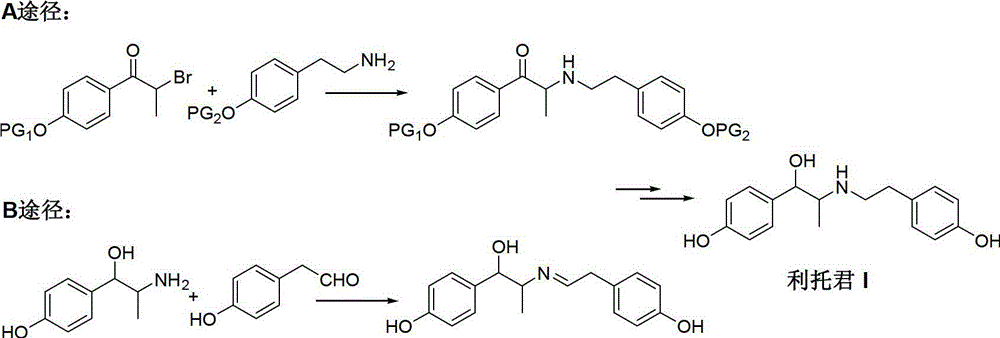
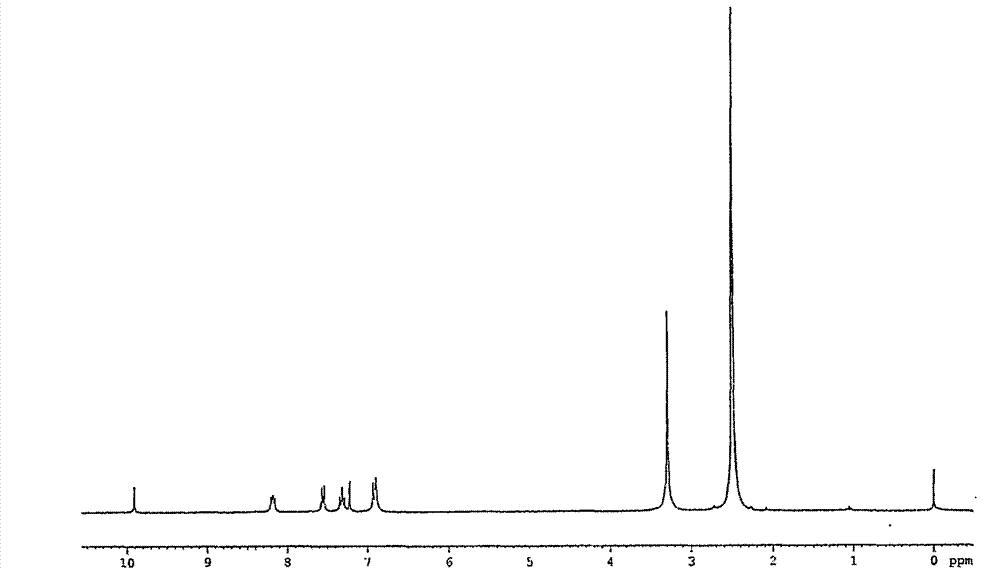
Preparation method of ritodrine
ActiveCN102976959ALow costImprove qualityOrganic compound preparationAmino-hyroxy compound preparationPropanolNitrite
The invention discloses a preparation method of ritodrine (1-(4-hydroxyphenyl)-2-[2-(4-hydroxyphenyl)ethylamino]propanol, I), which comprises the following steps: reacting 4-hydroxypropiophenone and alkyl nitrite to obtain an intermediate 2-oximino-4-hydroxypropiophenone (II); performing reduction reaction on the intermediate (II) to obtain 2-amino-1-(4-hydroxyphenyl)propanol hydrochloride (III); then, performing condensation reaction on the intermediate (III) and 4-hydroxyphenylacetaldehyde to generate a Schiff base intermediate (1-(4-hydroxyphenyl)-2-[2-(4-hydroxyphenyl)ethylimido]propanol, IV); and performing reduction reaction on the intermediate (IV) to obtain the ritodrine (I). The preparation method has high chemical selectivity and can be implemented without the protection of any functional group, so that the production cost and quality of the ritodrine (I) are greatly improved.
Owner:SUZHOU LIXIN PHARMA
Synthesis and application of antineoplastic 2-amino-3-cyano pyridine
InactiveCN102924372ARapid responseImprove responseOrganic active ingredientsOrganic chemistrySynthesis methodsStomach cancer
The invention provides a novel antineoplastic drug shown in a molecular formula (I). The synthesis method of the drug is simple and convenient, and the drug can be quickly and efficiently synthesized through microwave-assisted one-pot four-component reaction. The synthesis method comprises the following specific steps: dissolving 2 mmol of p-hydroxybenzaldehyde, 2 mmol of 4-fluoroacetophenone or 4-chloropropiophenone and 2 mmol of malononitrile in 1,4-dioxane; then adding 4 mmol of ammonium acetate; performing microwave (300W)-assisted heating to 120 DEG C, and reacting for 20 minutes; and adding ice water into the reaction mixture to precipitate a product, and then recrystallizing to obtain the product, namely 2-amino-3-cyano pyridine. The compound obtained by the invention is a novel antineoplastic drug shown in the molecular formula (I), and has the activity of obviously inhibiting the proliferation of liver cancer, stomach cancer, mammary cancer, pancreatic cancer, brain cancer, lung cancer and ovarian cancer cells.
Owner:CHINA PHARM UNIV +1
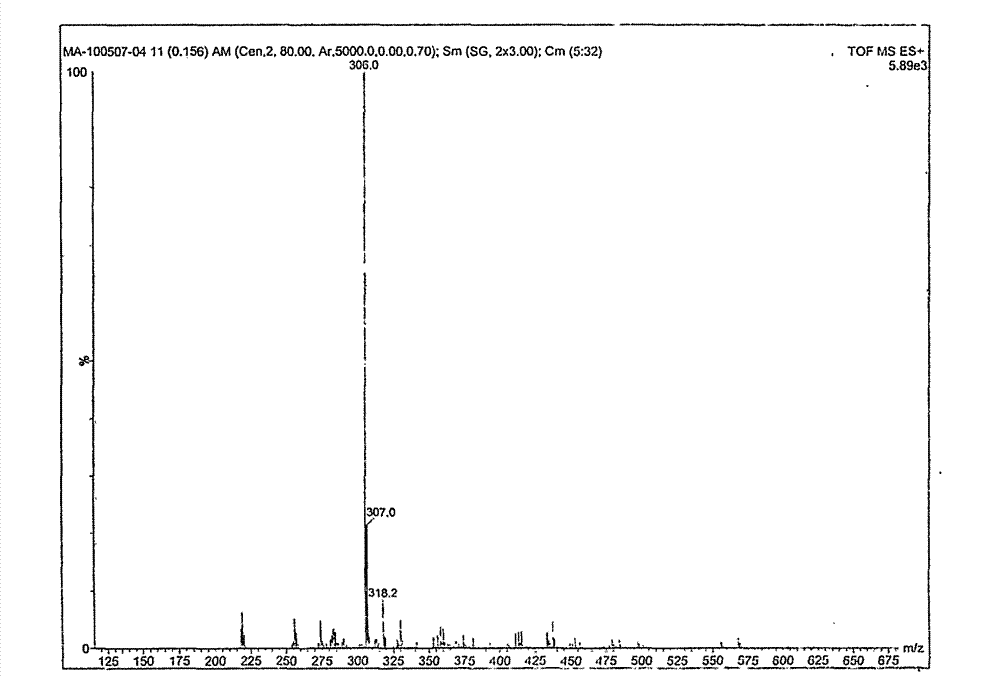
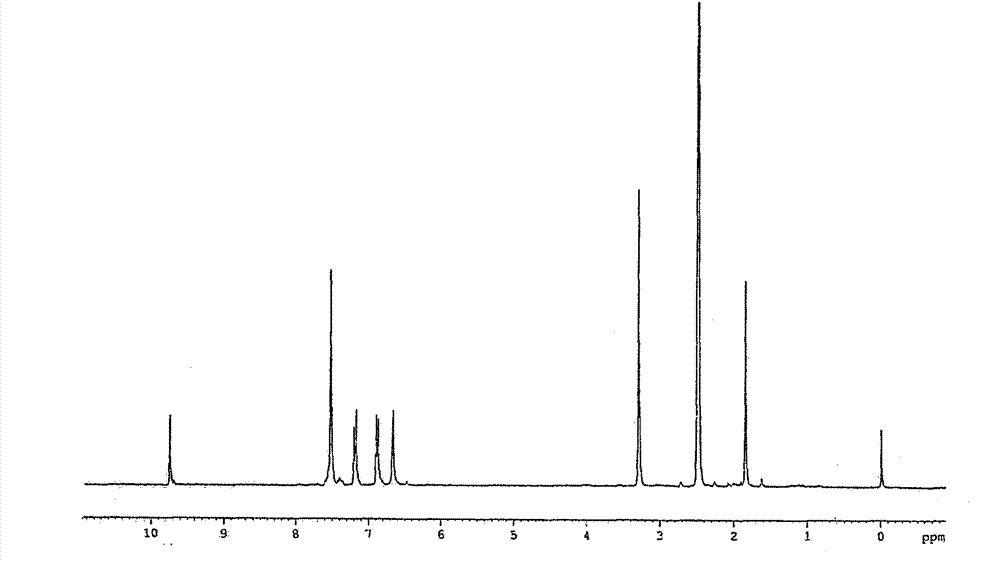
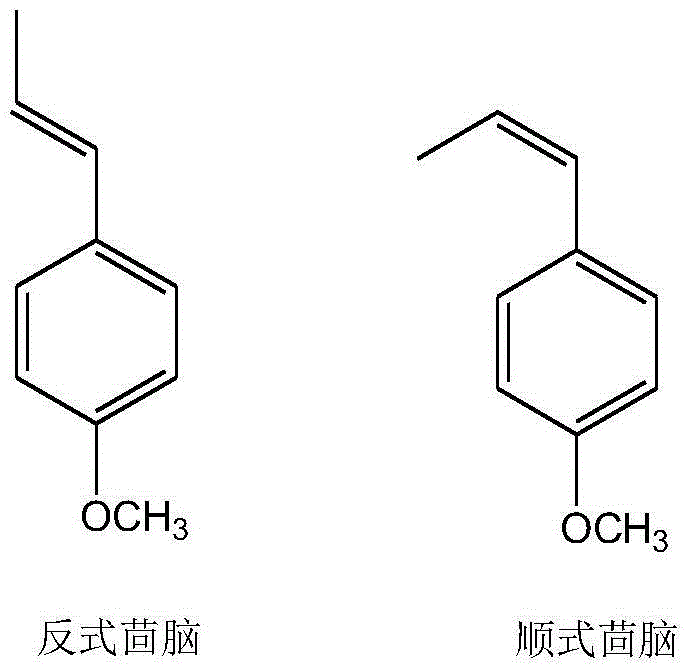
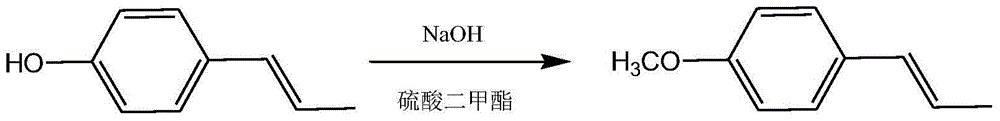
Preparation method of intermediate for synthesizing anise camphor
ActiveCN103951548AReduce manufacturing costImprove catalytic performanceOrganic chemistryOrganic compound preparationPropanolHydrogenation reaction
The invention discloses organic spice anise camphor and a production process of an intermediate of the anise camphor. The production process mainly comprises the following step: performing catalytic hydrogenation on methoxypropiophenone to generate 1-(4-methoxyphenyl) propanol. Cheap raney nickel is taken as a catalyst for the hydrogenation reaction in the production process, and consequently, the production cost is greatly reduced and the reaction time is shortened, and the product purity is also guaranteed; the product yield is more than 90%.
Owner:SUQIAN COSMOS CHEM
Hydrogel compositions
The invention relates to compositions comprising a hydrogel matrix, where the matrix comprises poly(ethylene glycol) dimethyacrylate (PEGDMA), an acrylate, such as methacrylic acid (M) and methyl methacrylate (MMA), as well as 2-hydroxy-2 methyl propiophenone (HMPP).
Owner:BECTON DICKINSON & CO
Method for asymmetrically catalyzing and synthesizing (R)-(+)-3-chlorine-phenylpropanol
ActiveCN102584536AReduce manufacturing costReduce storage costsOrganic compound preparationHydroxy compound preparationEthyl ChloridePropiophenones
The invention provides a method for asymmetrically catalyzing and synthesizing (R)-(+)-3-chlorine-phenylpropanol. The method is implemented as follows: metal hydroboron is taken as a reducing agent and organic acid is taken as an accessory ingredient to asymmetrically catalyze and reduce 3-chlorine-propiophenone; spiroborate and hydroboron are mixed by a tetrahydrofuran solvent, a certain quantity of organic acid is added and stirred until mixed solution is clear and transparent, tetrahydrofuran solution of the 3-chlorine-propiophenone is dropped slowly and stirred to reaction at the 50 degrees below zero to 30 DEG C, reaction liquid is purified to obtain the (R)-(+)-3-chlorine-phenylpropanol mono-spin-body. The optical purity of undetached (R)-(+)-3-chlorine-phenylpropanol is more than 90%e.e..
Owner:GUANGXI XINJING TECH +2
Light covering ink
The invention relates to light covering ink which comprises the following components in parts by mass: 35-50 parts of epoxy acrylate oligomer resin, 10-15 parts of hexanediol diacrylate, 1-4 parts of dibutyl phthalate, 15-30 parts of trimethylolpropane trimethyl propionate, 10-20 parts of ethylene glycol monobutyl ether, 5-10 parts of 2-hydroxyl-2-tolperisone, 2-4 parts of wax, 1-4 parts of diethanol amine, 25-30 parts of acetic ether, 0.1-0.8 parts of methyl silicone oil, and 10-15 parts of pure water. The light covering ink is high in friction resistance, heat resistance and adhesion resistance, prevents smudge and adhesion, can be piled, and has the advantages of non-yellowing and low odor.
Owner:苏州凹凸彩印厂
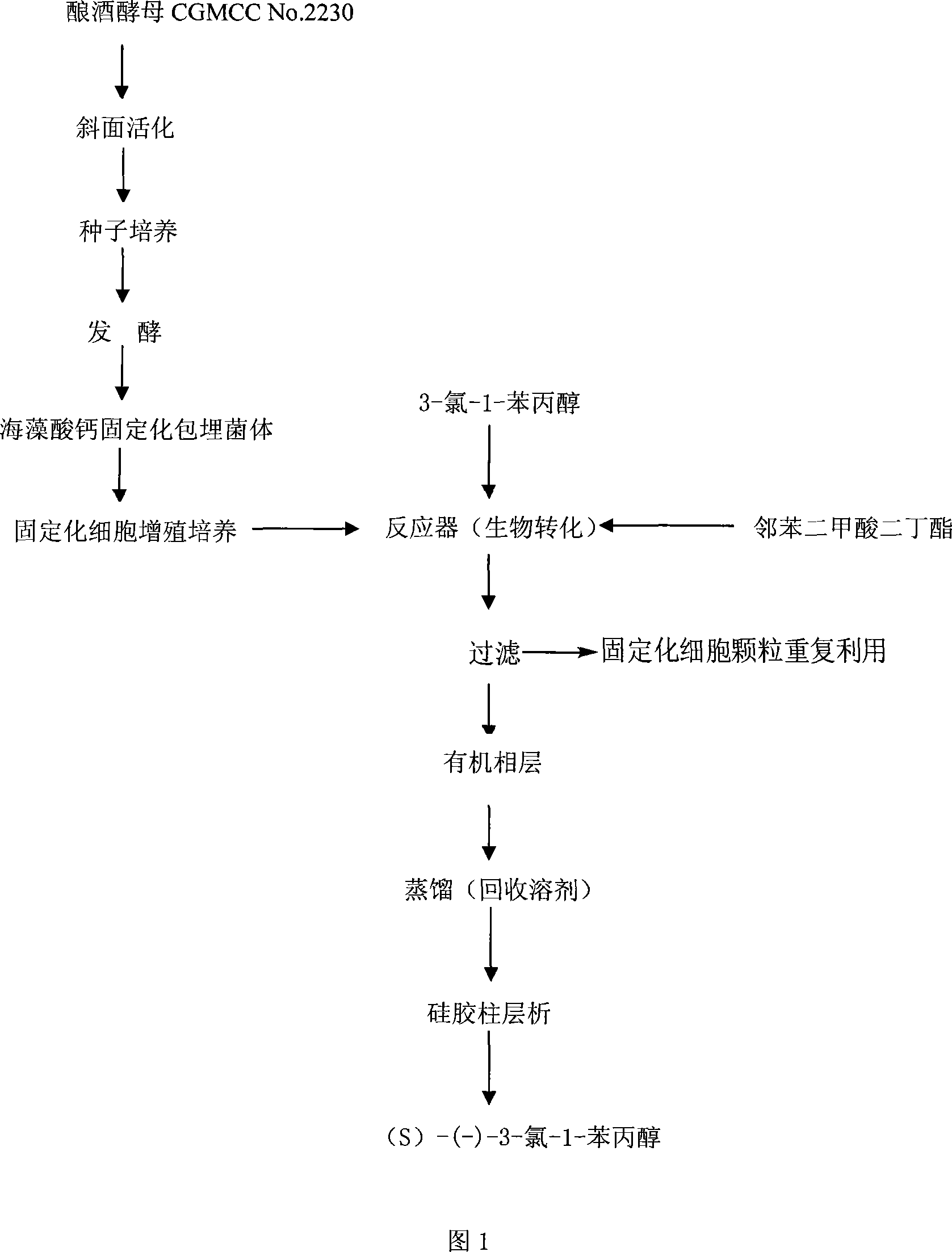
Use of saccharomyces cerevisiae in preparation of (S)-(-)-3-chlorine-1-phenylpropanol
InactiveCN101205548ALow costHigh stereoselectivityMicroorganism based processesChemical recyclingMicroorganismMicrobial transformation
The invention discloses an application of a novel microbial strain Saccharomyces cerevisiae CGMCC No.2230 in microbial transformation preparation of (S)-(-)-3-chlorine-1-phenylpropanols. The application of the invention is that: 3-chlorine-1-propiophenones are taken as substrates, and immobilized cell particles which are prepared by fermentation broth obtained after fermentation of the Saccharomyces cerevisiae strain CGMCC No.2230 are taken as biocatalysts; transformation reaction time of transformation liquid is 8 to 72 hours at the temperature of 25 to 30 DEG C in dibutyl phthalates, and the product S)-(-)-3-chlorine-1-phenylpropanols are obtained after separation and purification of the transformation liquid. The microbial transformation method has the advantages of friendly surroundings, mild reaction conditions, simple product separation, high substrate transformation ratio, good product purity and biocatalysts capable of being reused, and is suitable for commercial process.
Owner:ZHEJIANG UNIV OF TECH
High temperature resistant optical fiber and preparation method thereof
ActiveCN109180023AImprove toughnessHigh strengthGlass optical fibreOptical waveguide light guideUltrasound attenuationOligomer
The invention provides a high temperature resistant optical fiber and a preparation method thereof. The high temperature resistant optical fiber comprises an optical fiber core layer, a cladding layerand a double-layer coating, wherein, the coating material of an inner coating is composed of an acrylate oligomer, 2-hydroxyl-2-methyl propiophenone, vinyl tri(2-methoxy ethoxyl) silane and (2,4,6-trimethyl benzoyl)diphenyl phosphine xide; an outer coating is a high temperature resistant acrylic resin outer coating. The high temperature resistant optical fiber still maintains the reliability of optical transmission at a high temperature environment of 150DEG C for a long time, optical fiber coating has no aging phenomena of yellowing and blackening. The method is simple. The optical fiber transmission loss of the high temperature resistant optical fiber prepared at the wiredrawing speed of 2000-2200m / min, the attenuation value of the optical fiber at 1550nm is not more than 0.25dB / km, theadditional attenuation value of the optical fiber at environment of 150DEG C for a long time is not more than 0.05dB / km.
Owner:JIANGSU HENGTONG OPTICAL FIBER TECH +1
Preparation method of imidazo-[1,2-a] pyridine compound
The invention discloses a preparation method of an imidazo-[1,2-a] pyridine compound. The preparation method comprises the following steps of: (1) adding 2-aminopyridine compound, a propiophenone compound and a condensation catalyst to an organic solvent, and heating for condensation reaction, wherein an imine intermediate is obtained after complete reaction; (2) adding a copper catalyst, an oxidizing agent, alkali the imine intermediate obtained from the step (1) to the organic solvent, heating for ring closing reaction, and carrying out post processing to obtain the imidazo-[1,2-a] pyridine compound after the complete reaction. The preparation method disclosed by the invention has the advantages of wide source, cheapness and easiness for obtaining of used raw materials, namely the 2-aminopyridine compound and the propiophenone compound, is easy to operate without a complex post processing process and is suitable for the large-scale preparation of the compound.
Owner:ZHEJIANG UNIV
Synthetic method of alpha-amino aromatic ketone compound
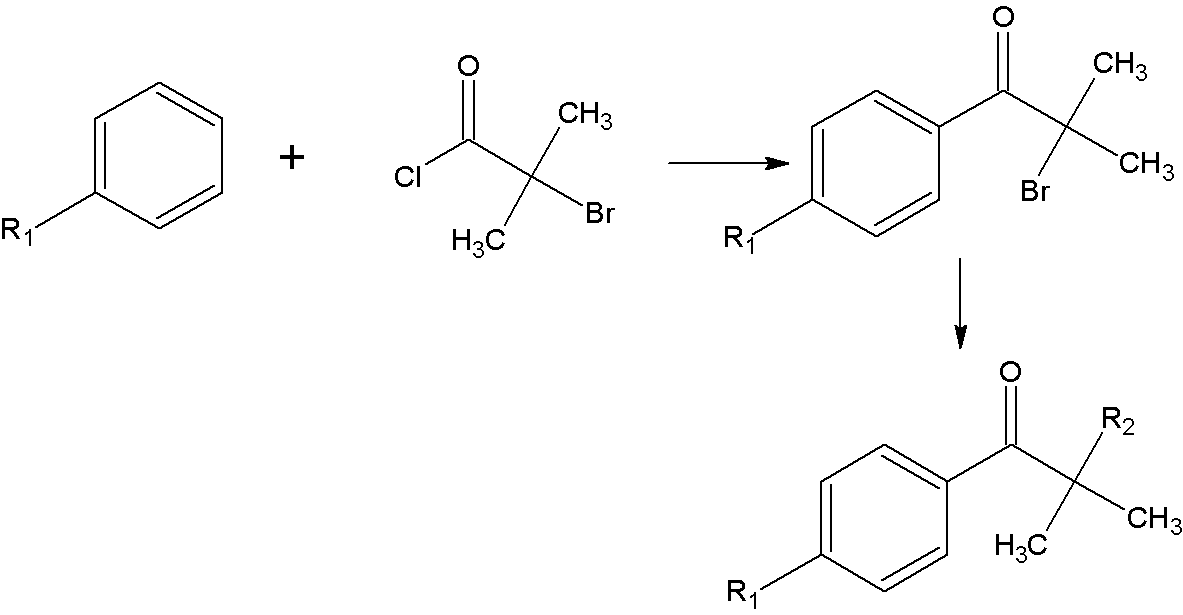
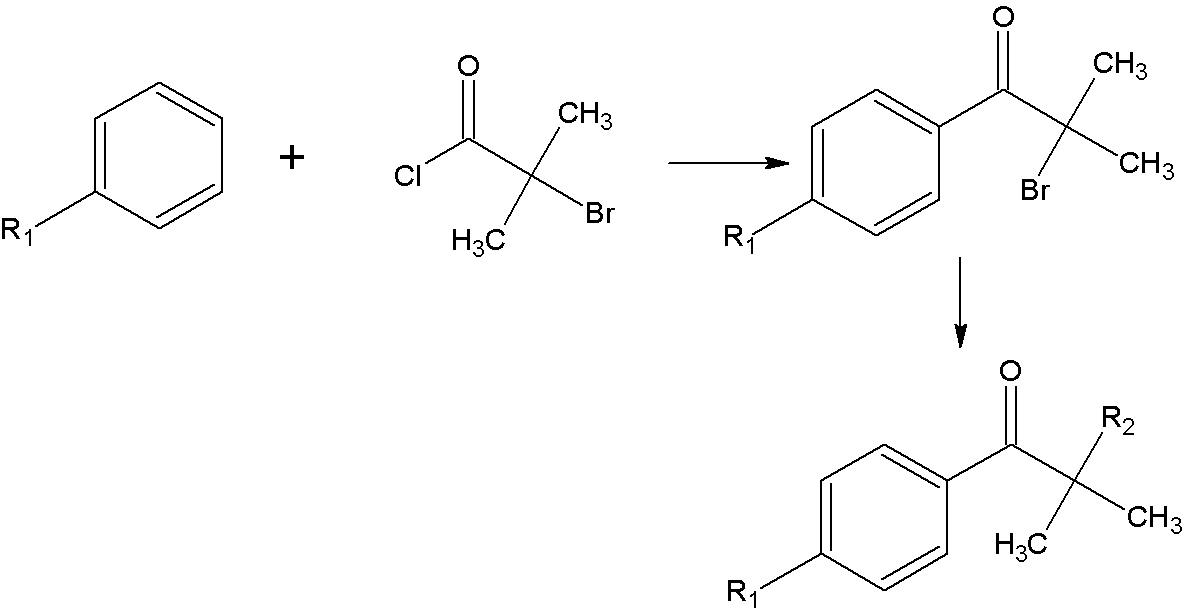
InactiveCN103242261AMeet the needs of industrial productionReduce pollutionOrganic compound preparationSulfide preparationPropiophenonesPhotoinitiator
The invention relates to a synthetic method of an alpha-amino aromatic ketone compound. The reaction method comprises step one, reacting benzene or substituted benzene and 2-bromo-isobutyl chloride with a water-insoluble solvent in the presence of a catalyst in the absence of water to generate an intermediate product 2-bromo-2-methyl-1-benzene or substituted propiophenone; and step two, reacting the intermediate product with a compound having a secondary amine structure under an action of an organic solvent and the catalyst, and performing aftertreatment of hydrolysis, extraction and the like to obtain the alpha-amino aromatic ketone compound of which the final yield is up to more than 80 percent and the purity is more than 97 percent. The synthetic method of the alpha-amino aromatic ketone compound is high in yield and product purity, low in environmental pollution and low in cost, and an industrial production requirement of alpha-amino aromatic ketone photoinitiator can be met, so that the possibility is provided for the industrial production of the product.
Owner:HUBEI UNIV OF TECH
Synthetic method for increasing yield of 5-chloro-1-indanone
InactiveCN111205175AHigh selectivityReduce generationOrganic compound preparationCarbonyl compound preparationPtru catalystOrganic synthesis
The invention provides a synthetic method for increasing the yield of 5-chloro-1-indanone, and belongs to the technical field of organic synthesis. The preparation method comprises the following steps: taking 3,4'-dichloropropiophenone as a raw material, adding an aprotic acid catalyst, then heating the mixture to a molten state, then adding a phase transfer catalyst, and carrying out reactions, wherein the aprotic acid catalyst is one of AlCl3, ZnCl2, InCl3, TiCl4, BF3, Fe(CF3SO3)3, Fe(CF3SO3)2 and gamma-alumina; the phase transfer catalyst is one of tetrabutyl ammonium bromide, tetrabutyl ammonium chloride, trioctyl methyl ammonium chloride, benzyl triethyl ammonium chloride, dodecyl trimethyl ammonium chloride, eighteen crowns six, fifteen crowns five, polyethylene glycol 400, polyethylene glycol 600 and polyethylene glycol 800. By adding the phase transfer catalyst, the selectivity of the Friedel-Crafts reaction is obviously improved, the side reaction is reduced, and the reactionyield is improved.
Owner:JINGBO AGROCHEM TECH CO LTD
Compounding method for beta-aryl propiophenone compound
ActiveCN107382640AMild reaction conditionsSimple and fast operationOrganic reductionOrganic compound preparationArylOrganic solvent
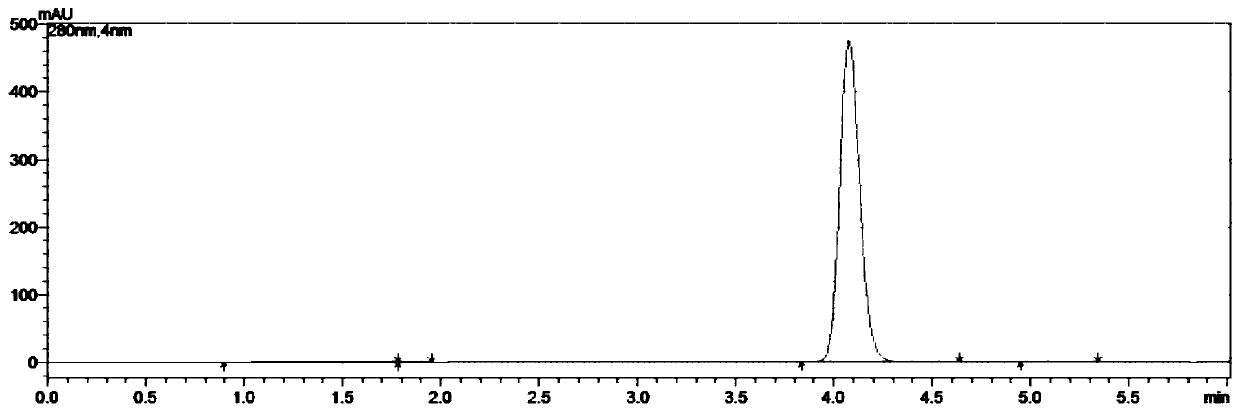
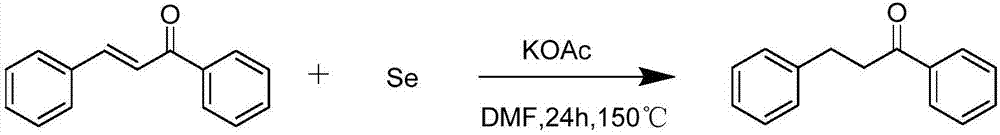
The invention discloses a compounding method for a beta-aryl propiophenone compound. The compounding method is characterized by taking chalcone compounds as reaction substrates, and selectively chemically reducing the carbon-carbon double bonds in the reaction substrates at 100-160 DEG C under the accelerating effect of inorganic alkaline and elemental selenium under the nitrogen condition in an organic solvent, thereby acquiring the beta-aryl propiophenone compound. The invention has the beneficial effects of mild reaction condition, simple and convenient operation, high functional tolerance, high reaction efficiency, high yield and purity, simple post-processing, reasonable price and suitability for large-scale industrial production.
Owner:WENZHOU UNIVERSITY
Self-repairing hydrogel microcapsule composite material and preparation method thereof, self-repairing lithium-sulfur battery cathode and battery
ActiveCN111193017AUniform sizeImprove liquidityPositive electrodesNon-aqueous electrolyte accumulator electrodesPolyvinyl alcoholElectrical battery
The invention discloses a self-repairing hydrogel microcapsule composite material, a preparation method thereof, a self-repairing lithium-sulfur battery cathode and a battery. The method comprises thesteps of: dissolving pyrrole and phytic acid in isopropanol to obtain a mixed solution A, and mixing the mixed solution A with an ammonium sulfate aqueous solution and deionized water to obtain a precursor solution B; dispersing 2-hydroxy-2-methyl propiophenone and trimethylolpropane ethoxylate triacrylate into absolute ethyl alcohol, and then heating and volatilizing to remove the absolute ethylalcohol, so as to obtain a mixed solution C; taking the precursor solution B, the mixed solution C and the polyvinyl alcohol aqueous solution as an inner phase, an outer phase and a driving phase; preparing a microcapsule by using a liquid drive coaxial flow focusing technology; and doping the self-repairing hydrogel microcapsule composite material into sulfur powder to be used as an active substance for preparing the cathode of a lithium-sulfur battery, and when an electrode plate cracks, releasing hydrogel in a microcapsule to precisely repair cracks of the cathode plate of the lithium-sulfur battery, so that good performance of the lithium-sulfur battery can be maintained.
Owner:ANHUI NORMAL UNIV
Novel hydrophobic modified nano-silicon material
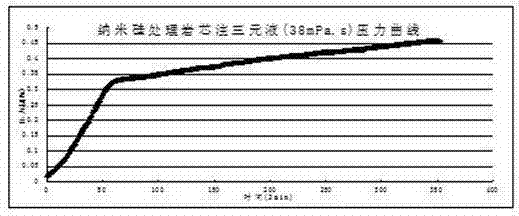
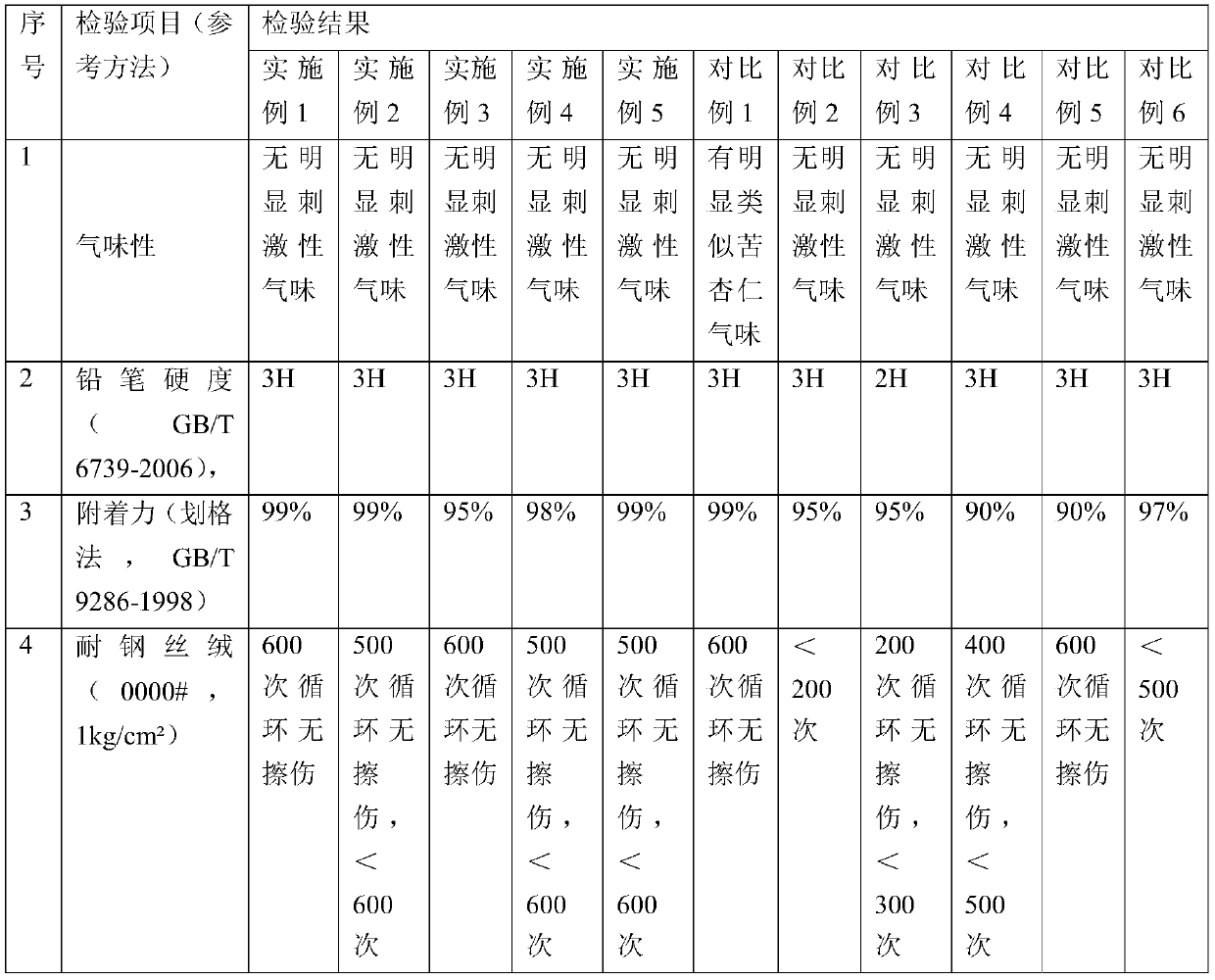
The invention discloses a novel hydrophobic modified nano-silicon material, aiming to solve the problem that the oil field injecting nano-silica material can not be applied to the polymer injection well and the alkaline-surfactant-polymer flooding injection well. The novel hydrophobic modified nano-silicon material is prepared according to the following steps: adding absolute alcohol into water-dispersible nano-silicon, dropwise adding the ethanol solution of silane coupling agent, and finally dropwise adding 1% of the ethanol solution of trimethoxyoctadecylsilane, wherein the mass of the ethanol solution of silane coupling agent is 0.5-2% of that of the nano-silicon. The novel hydrophobic modified nano-silicon material can be uniformly dispersed into a variety of solvent media including water, isopropyl alcohol, ethanol, ethylene glycol, xylene and acetone and can form a super-hydrophobic layer on the surface of the rock core.
Owner:BC P INC CHINA NAT PETROLEUM CORP +2
Ultraviolet (UV) cured paint and preparation method thereof
InactiveCN103525264AGood coating performanceMeet the requirements of ROHS directiveEpoxy resin coatingsMethacrylateGlycidyl methacrylate
The invention relates to an ultraviolet (UV) cured paint. The UV cured paint is prepared from the following raw materials in part by weight: 50-60 parts of epoxy acrylate, 20-25 parts of methacrylate, 6-8 parts of 2-hydroxyl-2-methyl-propiophenone, 10-12 parts of dimethylaminoethyl methacrylate, 1-2 parts of polyoxyethylene stearate, 10-12 parts of dipentaerythritol hexaacrylate, 3-5 parts of ethylene glycol monoethyl ether acetate, 1-2 parts of a silane coupling agent KH-550, 3-4 parts of an accelerator DM, 2-3 parts of acrylic acid-4-polyhydroxybutyrate, 1-2 parts of glycidyl methacrylate, 2-3 parts of bis tetradecene ester, 1-2 parts of an antioxidant DSTP (disteaxylthiodipropionate), and 4-5 parts of a dispersing aid. The UV paint is suitable for priming paints of hardware, zinc alloy, aluminium alloy, stainless steel, and vacuum plating coating UV, has lasting performance and strong universality, and meets the ROHS (Restriction Of Hazardous Substances) requirements.
Owner:安徽蓝柯复合材料有限公司
UV scratch-resistant agent of organic-inorganic composite system and preparation method and application of UV scratch-resistant agent
InactiveCN110577796ASmall smellImprove adhesionPolyurea/polyurethane coatingsPhosphateInorganic compound
The invention discloses a UV scratch-resistant agent of an organic-inorganic composite system and a preparation method and an application of the UV scratch-resistant agent. The UV scratch-resistant agent comprises the following raw materials by the weight percentage: 26-34% of a urethane acrylate oligomer, 2.4-6.4% of a nano silicon dioxide dispersion liquid, 0.3% of an adhesion promoter, 44-52% of a solvent, 0.3% of a reactive leveling agent, 3% of a macromolecular photoinitiator and 12% of an acrylate reactive diluent, wherein the sum of the weight percentages of the raw materials is 100%. The urethane acrylate oligomer comprises a urethane acrylate oligomer with six functional groups; the adhesion promoter comprises a phosphate modified acrylate oligomer; the leveling agent comprises acrylic acid modified polysiloxane; the macromolecular photoinitiator comprises 2-hydroxy-4-(2-hydroxyethyl)-2-methyl propiophenone. After coating the surface of a window film and being cured into a film, the scratch-resistant agent has the characteristics of low odor, high hardness, good adhesive force, excellent wear resistance and the like, and meets the index requirements of the window film market on film hardening treatment.
Owner:西安航天三沃化学有限公司
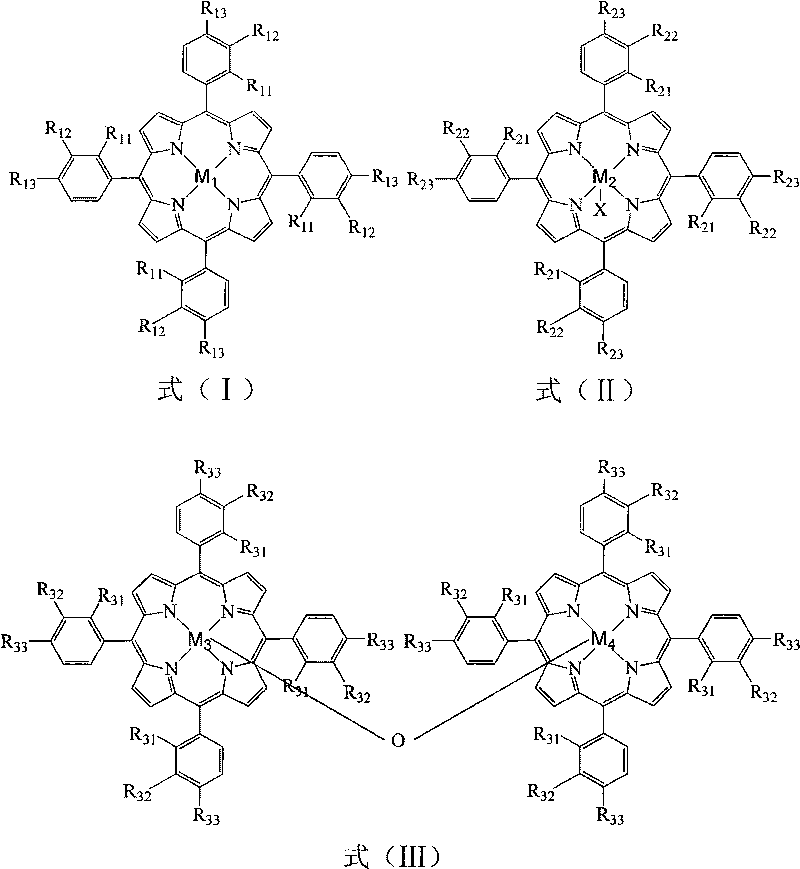
Method for preparing propiophenone by biomimetic catalytic oxidation of n-propylbenzene with oxygen
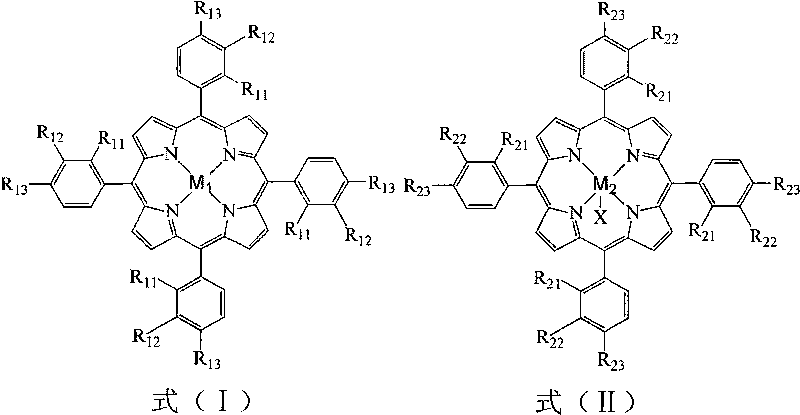
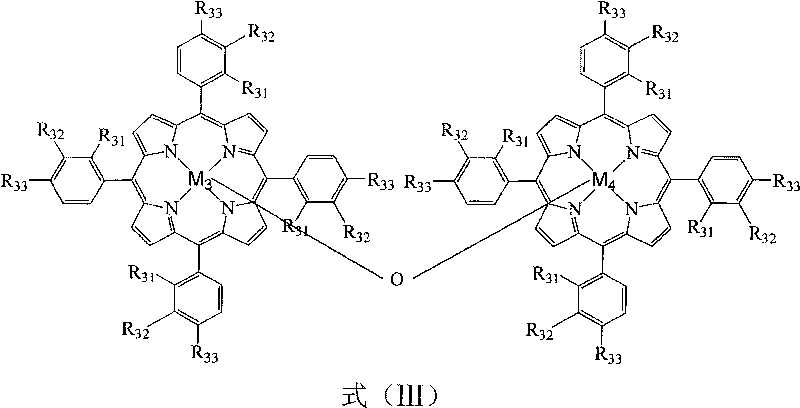
InactiveCN101759540AReduce pollutionReduce manufacturing costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic oxidationPorphyrin
The invention relates to a method for preparing propiophenone by biomimetic catalytic oxidation of n-propylbenzene with oxygen, comprising the following steps: taking n-propylbenzene as the raw material, selecting any one of 1-30ppm of mononuclear metalloporphyrin and mu-oxo-dinuclear metalloporphyrin or the composition of the two substances as the catalyst under normal pressure and in the absence of solvents, introducing oxygen at the flow rate of 10-60mL / min, initiating reaction at 140-160 DEG C and then carrying out reaction at 70-110 DEG C for 4-10h, thus obtaining the propiophenone. In the method, the way of high temperature quick initiation and low temperature reaction is adopted, thus minimizing the reaction initiation time, greatly shortening the reaction time, improving the reaction efficiency, reducing the energy consumption, lowering the operation cost and improving the reaction safety.
Owner:BEIJING UNIV OF TECH
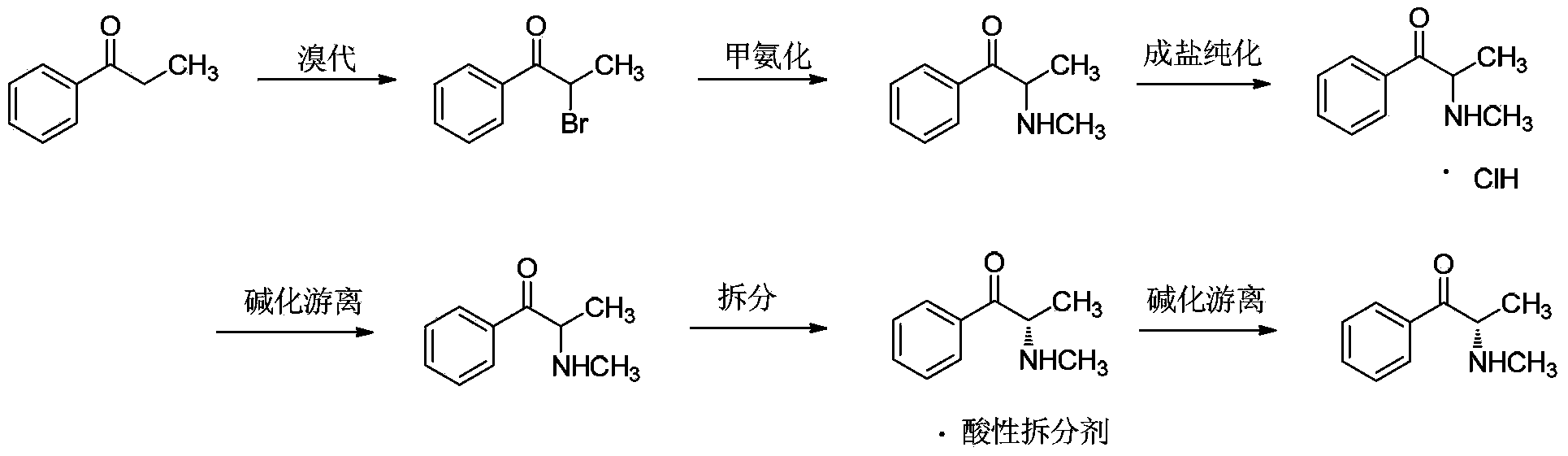
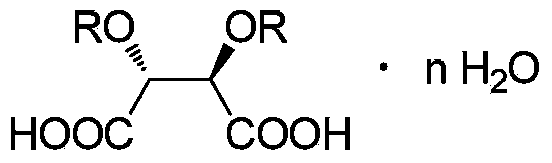
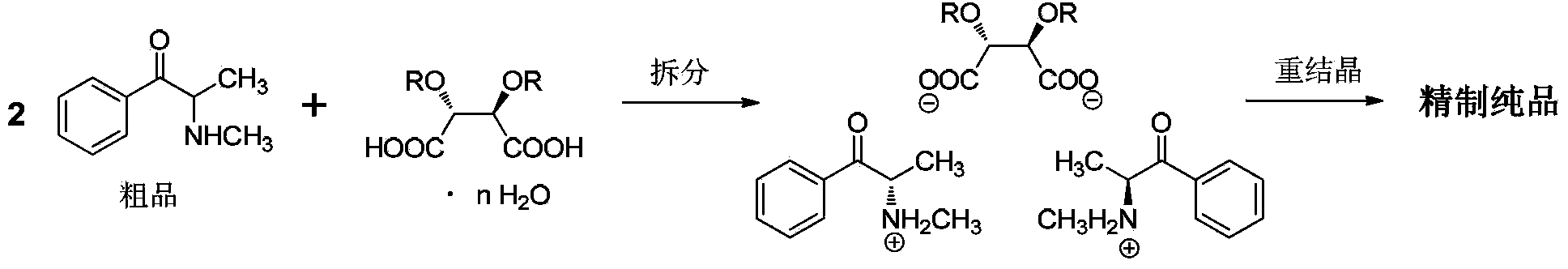
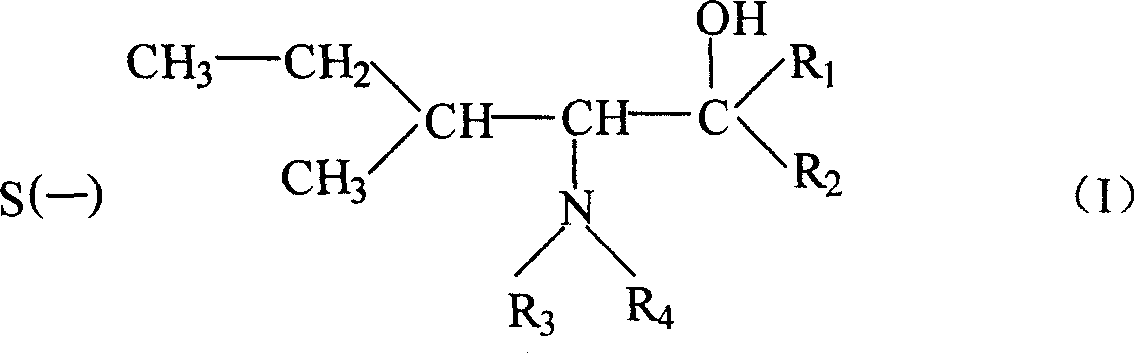
Preparation method for (S)-(-)-alpha-methylaminopropiophenone
InactiveCN104119240AEasy to operateLow costOrganic chemistryOrganic compound preparationPhenacylBiochemical engineering
The invention belongs to the field of medicinal chemical engineering, and relates to a preparation method for (S)-(-)-alpha-methylaminopropiophenone. The invention also relates to a composition, application thereof and a method of preparing ephedrine or pseudoephedrine. Concretely, the invention discloses preparation and a resolution purification technology of alpha-methylaminopropiophenone. Propiophenone or bromopropiophenone is taken as a raw material and is subjected to continuous synthesis, and resolution and purification of optically-pure alpha-methylaminopropiophenone are directly performed without performing synthesis and separation of alpha-methylaminopropiophenone hydrochloride, and the final product is obtained in the form of a [(S)-(-)-alpha-methylaminopropiophenone]2.(2R,3R)-dibenzoyltartaric acid derivative. Compared with the prior art, the preparation method is relatively simple in operation, relatively low in cost and relatively high in reaction yield and resolution efficiency, and all solvents are recyclable.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![2-amido-2-[2-(4-alkylphenyl)ethyl]-1,3-methyl glycol preparation method 2-amido-2-[2-(4-alkylphenyl)ethyl]-1,3-methyl glycol preparation method](https://images-eureka.patsnap.com/patent_img/6951d141-6d8e-4d5b-b5a8-4293d3c0a10a/C2005100957660002C1.PNG)
![2-amido-2-[2-(4-alkylphenyl)ethyl]-1,3-methyl glycol preparation method 2-amido-2-[2-(4-alkylphenyl)ethyl]-1,3-methyl glycol preparation method](https://images-eureka.patsnap.com/patent_img/6951d141-6d8e-4d5b-b5a8-4293d3c0a10a/C2005100957660002C2.PNG)
![2-amido-2-[2-(4-alkylphenyl)ethyl]-1,3-methyl glycol preparation method 2-amido-2-[2-(4-alkylphenyl)ethyl]-1,3-methyl glycol preparation method](https://images-eureka.patsnap.com/patent_img/6951d141-6d8e-4d5b-b5a8-4293d3c0a10a/C2005100957660002C3.PNG)


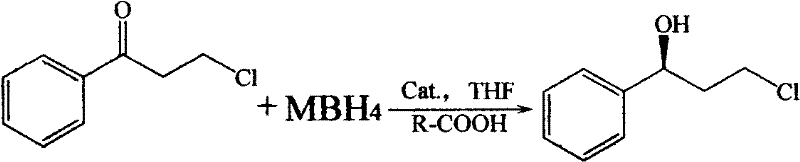




![Preparation method of imidazo-[1,2-a] pyridine compound Preparation method of imidazo-[1,2-a] pyridine compound](https://images-eureka.patsnap.com/patent_img/389706a4-af23-465b-a498-5fcd75391a4f/BDA0000378053600000021.PNG)
![Preparation method of imidazo-[1,2-a] pyridine compound Preparation method of imidazo-[1,2-a] pyridine compound](https://images-eureka.patsnap.com/patent_img/389706a4-af23-465b-a498-5fcd75391a4f/BDA0000378053600000022.PNG)
![Preparation method of imidazo-[1,2-a] pyridine compound Preparation method of imidazo-[1,2-a] pyridine compound](https://images-eureka.patsnap.com/patent_img/389706a4-af23-465b-a498-5fcd75391a4f/BDA0000378053600000023.PNG)