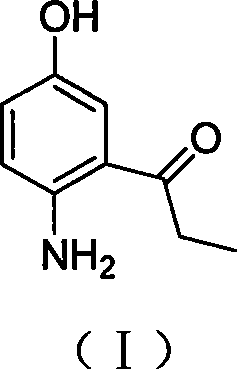
2-amino-5-hydroxypropiophenone preparation method
A technology of hydroxypropiophenone and amino, which is applied in the field of preparation of intermediate 2-amino-5-hydroxypropiophenone, can solve the problems of inability to realize industrial production, pollution of three wastes, harsh reaction conditions, etc., and achieve good industrialization prospects and environmental friendliness , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
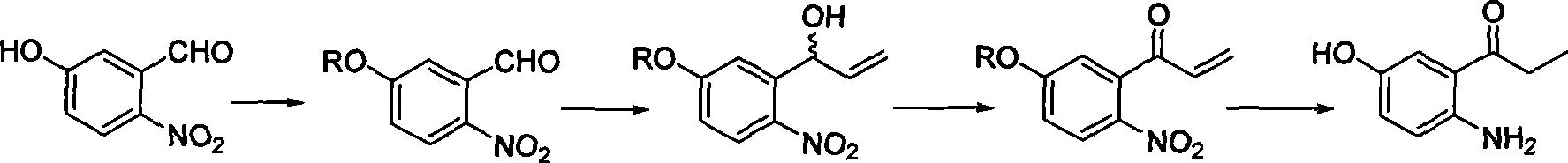
[0083] Compound 2-nitro-5-hydroxybenzaldehyde (II) 10.0g (59.88mmol), dimethyl sulfate 18.86g (0.15mol) and water (80ml) were placed in a reaction flask, under normal pressure, below 40 React at ℃, continuously add 30% potassium hydroxide solution dropwise during the reaction to control the pH of the reaction solution to 9~10 (the molar ratio of compound (II) to NaOH is 1:1.2~8.5), after the pH of the reaction solution is stable, The temperature was raised to 40° C. to continue the reaction for 4 hours (the total reaction time was 5-10 hours). The reactant was filtered, the filter cake was washed with water until neutral, and dried to obtain 9.53 g of white needle-like solid 2-nitro-5-methoxybenzaldehyde (III), melting point 83.1-83.8°C, yield 85.1%, content ≥ 93.9 %(GC / MS)(m / z)=181.
Embodiment 2
[0085] Compound 2-nitro-5-hydroxybenzaldehyde (II) 10.0g (59.88mmol) and acetone (150ml) were placed in a reaction flask, and potassium carbonate 9.93g (0.1mol ), and then gradually added 10.21 g (71.86 mmol) of iodomethane dropwise. After the dropwise addition, the temperature rose to 60° C. to continue the reaction for 4.5 hours (the total reaction time was 5 to 10 hours). After the reaction solution was cooled to room temperature, the solvent was evaporated under reduced pressure, 135ml of ethyl acetate was added, and the organic layer was washed with 10% sodium hydroxide solution (10ml×2) and water (15ml×2). After drying over anhydrous sodium sulfate, ethyl acetate was recovered to obtain 9.87 g of white solid 2-nitro-5-methoxybenzaldehyde (III), with a melting point of 84.0-84.9° C., a yield of 91.8%, and a content of ≥96.9% (GC / MS) (m / z) = 181.
[0086] (two) the preparation of 2-nitro-5-methoxybenzonitrile (V):
[0087] Example 1
[0088] Method A is a two-step meth...
Embodiment 3
[0096] Method B is a one-step method, 2-nitro-5-methoxybenzaldehyde (III) directly produces 2-nitro-5-methoxybenzonitrile (V) through step C:
[0097] Compound 2-nitro-5-methoxybenzaldehyde (III) 7.0g (38.67mmol), hydroxylamine hydrochloride 2.9g (42.54mmol), sodium formate 6.16g (0.15mol) and 85% formic acid 40ml were placed in the reaction flask, The reaction temperature was 70-135°C, and the reaction was stirred under reflux for 0.5 hours. After the reaction was completed, the reaction solution was cooled and poured into ice water, neutralized with 10% sodium hydroxide solution, extracted with ethyl acetate (30ml×3), the organic layer was washed with water (15ml×2), and dried over anhydrous sodium sulfate Afterwards, reclaim ethyl acetate, obtain compound 2-nitro-5-methoxybenzonitrile (V) 5.03g, fusing point 148.6~149.5 ℃, yield 75.4%, content≥93.8% (GC / MS) (m / z)=178.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com