Preparation method of rosuvastatin
A technology of rosuvastatin and fluorophenyl, which is applied in the field of preparation of pharmaceutical compounds, can solve problems such as high equipment requirements, large pollution, and high cost, and achieve the effects of shortening reaction steps, reducing production costs, and increasing production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
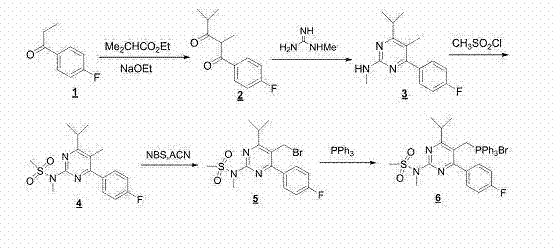
[0042] The preparation method of rosuvastatin is made up of following five steps:
[0043] Step 1: Preparation of 1-(4-fluorophenyl)-2,4-dimethylpentane-1,3-dione
[0044] Under nitrogen protection, add 153.0g (1.0 mol) of 4-fluoropropiophenone and 1,500ml of 1,4-dioxane into a 5000ml three-neck flask respectively, add 80g of sodium hydride (60% NaH, 2.0 mol) under stirring, and stir After uniformity, 139.2g (1.2mol) of ethyl isobutyrate was slowly added dropwise, and gas was generated during the dropwise addition; after the dropwise addition, the temperature was raised to 75-80°C, and the reaction was carried out for 5 hours. The reaction liquid was cooled to 0°C, and kept at 0 ~5°C, 2000ml of 2M hydrochloric acid solution was added dropwise, the reaction solution was extracted with ethyl acetate (3×500ml), the organic layer was washed once with 500ml of saturated brine, concentrated under reduced pressure to obtain the oily substance 1-(4-fluorophenyl)- 2,4-dimethylpentane-...
Embodiment 2
[0054] Embodiment 2 is basically the same as Embodiment 1, the only difference is that step 1 is different. Step 1 of this embodiment is specifically as follows:
[0055] Preparation of 1-(4-fluorophenyl)-2,4-dimethylpentane-1,3-dione
[0056] Under the protection of nitrogen, add 91.8g (0.6mol) of 4-fluoropropiophenone and 700ml of absolute ethanol to a 2000ml three-necked bottle respectively, and add 87.0g (1.2mol) of sodium ethylate under stirring, after stirring evenly, slowly add isobutyl Ethyl acetate 83.9g (0.7mol), after the dropwise addition, heat up to reflux, react for 7h, cool the reaction solution to 0°C, keep 0-5°C, add 2M hydrochloric acid solution dropwise, adjust the pH value to 5-6, The reaction solution was extracted with ethyl acetate (3×0.5L), the organic layer was washed once with 500ml of saturated brine, and concentrated under reduced pressure to obtain the oily substance 1-(4-fluorophenyl)-2,4-dimethylpentane-1 , 3-diketone 128.6g. Yield 96%, HPLC p...
Embodiment 3
[0058] Embodiment 3 is basically the same as Embodiment 1, the only difference is that step 4 is different. Step 4 of this embodiment is specifically as follows:
[0059] Preparation of N-(5-(bromomethyl)-4-(4-fluorophenyl)-6-isopropylpyrimidin-2-yl)-N-methylmethanesulfonamide
[0060] Under the protection of nitrogen, add N-(5-methyl)-4-(4-fluorophenyl)-6-isopropylpyrimidin-2-yl)-N-methylmethanesulfonate to a 50ml three-necked flask Amide 3.4g (10mmol) and N-bromosuccinimide 2.3g (13mmol), acetonitrile 20ml, the reaction solution with a λ=310nm light source, light for 5h, 25ml of water was added to the reaction solution, and the mixture was extracted with dichloromethane ( 3×10ml), the organic layers were combined, washed once with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 4.0g of the crude product, which was recrystallized with petroleum ether: methyl tert-butyl ether = 1:1 to obtain a white solid N-(5 -(Bromomethyl)-4-(4-fluoropheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com