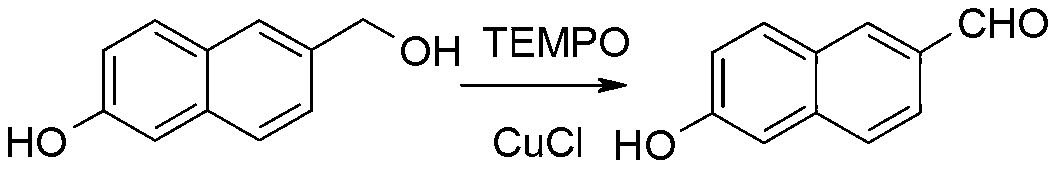
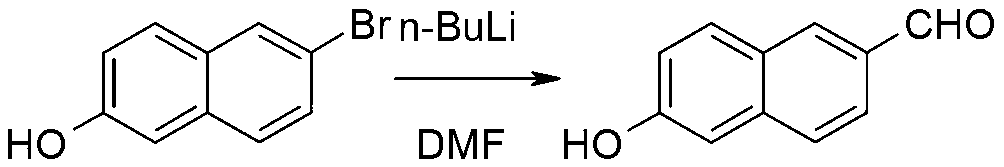
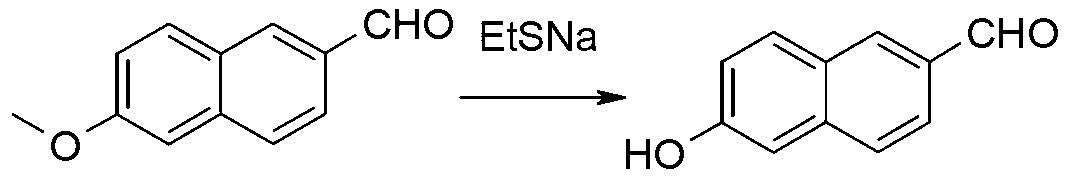
Synthetic method of 6-hydroxy-2-naphthaldehyde
A synthesis method and naphthalene formaldehyde technology are applied in the chemical field, can solve the problems of difficult synthesis, high cost, expensive oxidant TEMPO and the like, and achieve the effects of simple reaction conditions and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Step A: In the reaction vessel, add 288g of ethyl naphthol and 1300mL of glacial acetic acid, cool to 10-20°C, and control this temperature, slowly add 704g of bromine dropwise, after the addition is complete, heat up to 50-60°C for reaction After 2 hours, the reaction is complete, add 124g of iron powder, keep warm at 50-60°C for 2 hours, filter while hot, and concentrate the filtrate to dryness to obtain 440g of 6-bromo-2-naphthol, HPLC purity 97.2%, molar yield 95.8 %.
[0032] Step B: In the reaction vessel, put 334.5g of 6-bromo-2-naphthol, add 1500ml of water, add 72g of sodium hydroxide after stirring, stir evenly, control the temperature between 0 and 10°C, slowly add 246g of disulfuric acid disulfide After the addition of methyl ester was completed, the temperature was raised to 20-30°C to react for 4 hours. After the reaction was completed, extract 3 times with ethyl acetate, each time with 750ml of ethyl acetate, combine the ethyl acetate, add 225g of anhydro...
Embodiment 2
[0036] Step A: In the reaction vessel, add 432g of ethyl naphthol and 1950mL of glacial acetic acid, cool to 10-20°C, and control the temperature between 10-20°C, slowly add 960g of bromine dropwise, after the addition is complete, heat up to 50-60°C ℃ for 2 hours, the reaction is complete, add 219g of iron powder, keep warm at 50-60 ℃ for 2 hours, filter while hot, and concentrate the filtrate to dryness to obtain 666g of 6-bromo-2-naphthol, HPLC purity 97.0%, molar yield 96.5% .
[0037] Step B: Put 624.4g of 6-bromo-2-naphthol into the reaction vessel, add 2800ml of water, add 168g of sodium hydroxide after stirring, stir evenly, control the temperature between 0 and 10°C, and slowly add 705.6g of sulfuric acid dropwise After the addition of dimethyl ester is completed, the temperature is raised to 20-30°C for 4 hours. After the reaction is complete, extract with ethyl acetate for 3 times, each time with 1400ml of ethyl acetate, combine the ethyl acetate, add 420g of anhydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com