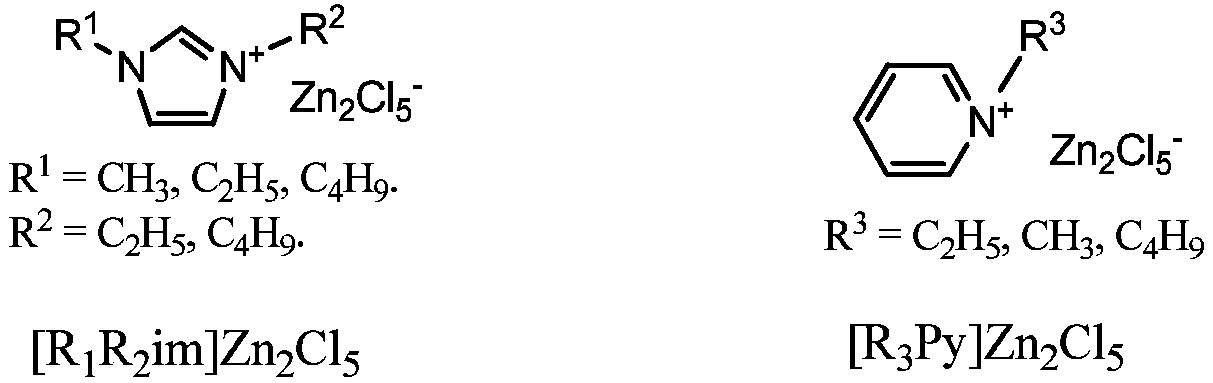Method for preparation of cyclic myrac aldehydes from myrac aldehyde catalyzed by zinc-containing ionic liquid
A technology of ionic liquids and zinc ions, which is applied in the field of zinc-containing ionic liquids to catalyze the conversion of citrus green aldehydes to prepare ring citrus green aldehydes, can solve problems such as environmental pollution, harsh reaction conditions, and waste acid production, and achieve high atom economy, The effect of fast response and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of ionic liquid catalyst: refer to literature (Li C, Zhao Z.Adv.Synth.Catal.2007, 349,1847; Webb P B, Sellin M F, Kunen T E, et al.J.Am.Chem.Soc.2003,125 , 15577; NodaA, Watanabe M.Electrochimica Acta.2000,45,1265; Sheldrake G N, Schleck D.GreenChem.2007,9,1044.) and patent (WO 00 / 16902) prepared and purified nine Lewis ionic liquids, Respectively: 1-methyl-3-butylimidazole-zinc chloride [C 4 mim]Zn 2 Cl 5 , 1-methyl-3-ethylimidazole-zinc chloride [C 2 mim]Zn 2 Cl 5 , 1-ethyl-3-butylimidazole-zinc chloride [C 4 C 2 im]Zn 2 Cl 5 , 1-ethyl-3-ethylimidazole-zinc chloride [C 2 C 2 im]Zn 2 Cl 5 , 1-butyl-3-butylimidazole-zinc chloride [C 4 C 4 im]Zn 2 Cl 5 , 1-butyl-3-ethylimidazole-zinc chloride [C 2 C 4 mim]Zn 2 Cl 5 , picoline-zinc chloride [MPy]Zn 2 Cl 5 , ethylpyridine-zinc chloride [C 2 Py]Zn 2 Cl 5 , butylpyridine-zinc chloride [C 4 Py]Zn 2 Cl5 , for the implementation of the patent of the present invention.
Embodiment 2-13
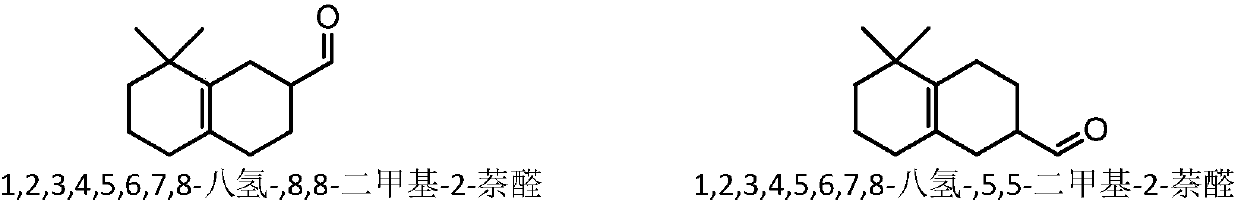
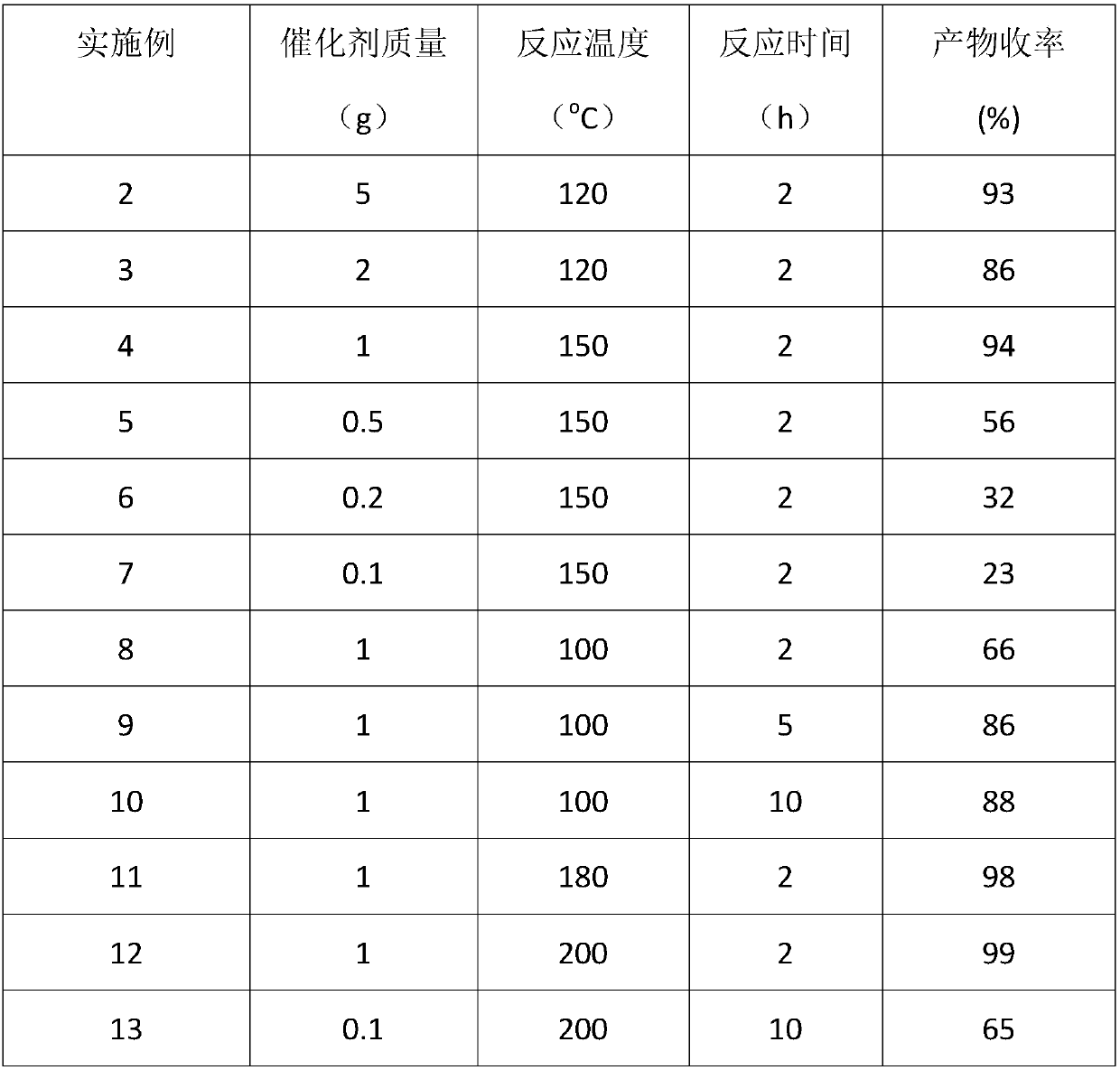
[0024] [C 4 mim]Zn 2 Cl 5 Catalytic selective cyclization of para-citrus aldehyde to prepare citrus aldehyde: 10 g of the reaction substrate and a certain mass of [C 4 mim]Zn 2 Cl 5 Catalysts were added to the reactor respectively, sealed and replaced with nitrogen five times, the initial pressure of nitrogen was 0.1MPa, the temperature was raised to 100-200°C, and the stirring reaction was carried out at a speed of 1000 rpm for 1-10h. After the reaction was completed, it was cooled to room temperature, and the reaction mixture was separated into two layers. The supernatant was poured and then sampled for analysis. The remaining ionic liquid catalyst in the lower layer was washed three times with n-hexane and directly used for the next reaction (see Examples 14-18). Qualitative analysis of the product was carried out by GC-MS technology and standard sample control, and quantitative analysis was realized by gas chromatography internal standard method. The reaction result...
Embodiment 14-18
[0028] [C 4 mim]Zn 2 Cl 5 The repeated use experiment results of catalyzing the selective cyclization of p-citrus aldehyde to prepare cyclocitrus green agent: 10g of p-citrus aldehyde and 1g of [C 4 mim]Zn 2 Cl 5 Catalysts were added to the reactor respectively, sealed and replaced with nitrogen five times, and the initial pressure of nitrogen was 0.1 MPa, the temperature was raised to 150°C, and the stirring reaction was carried out at a speed of 1000 rpm for 4 hours. After the reaction was completed, it was cooled to room temperature, and the reaction mixture was separated into two layers. The supernatant was poured and then sampled for analysis. The remaining ionic liquid catalyst in the lower layer was washed three times with n-hexane and directly used for the next reaction, and was reused five times. Qualitative analysis of the product was carried out by GC-MS technology and standard sample control, and quantitative analysis was realized by gas chromatography intern...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com