Preparation process of 3,6-dihydroxy-2-naphthaldehyde
A dihydroxynaphthalene and dihydroxyl technology, applied in the field of organic synthesis, can solve problems such as long synthesis steps and unsatisfactory yield, and achieve the effects of shortening steps, reducing waiting time for reaction, and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
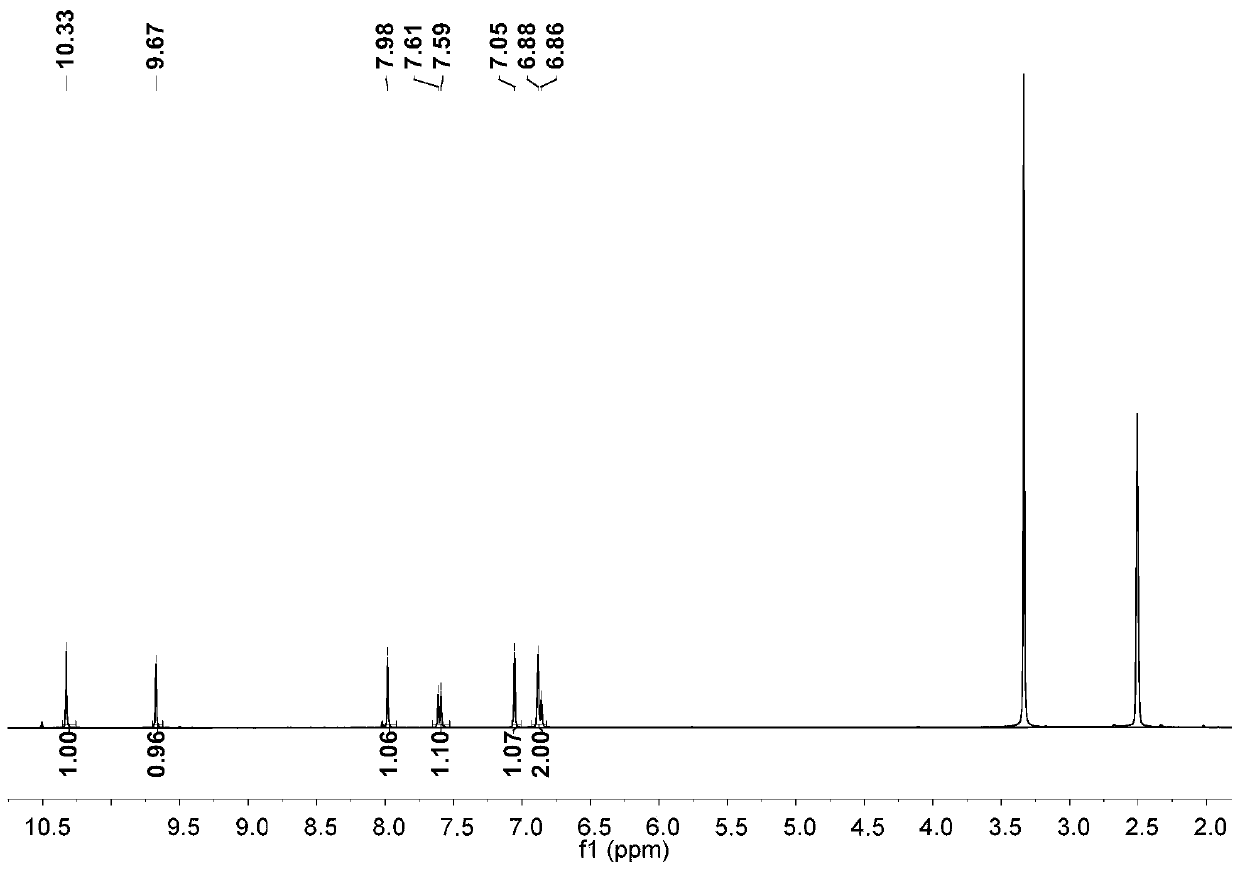
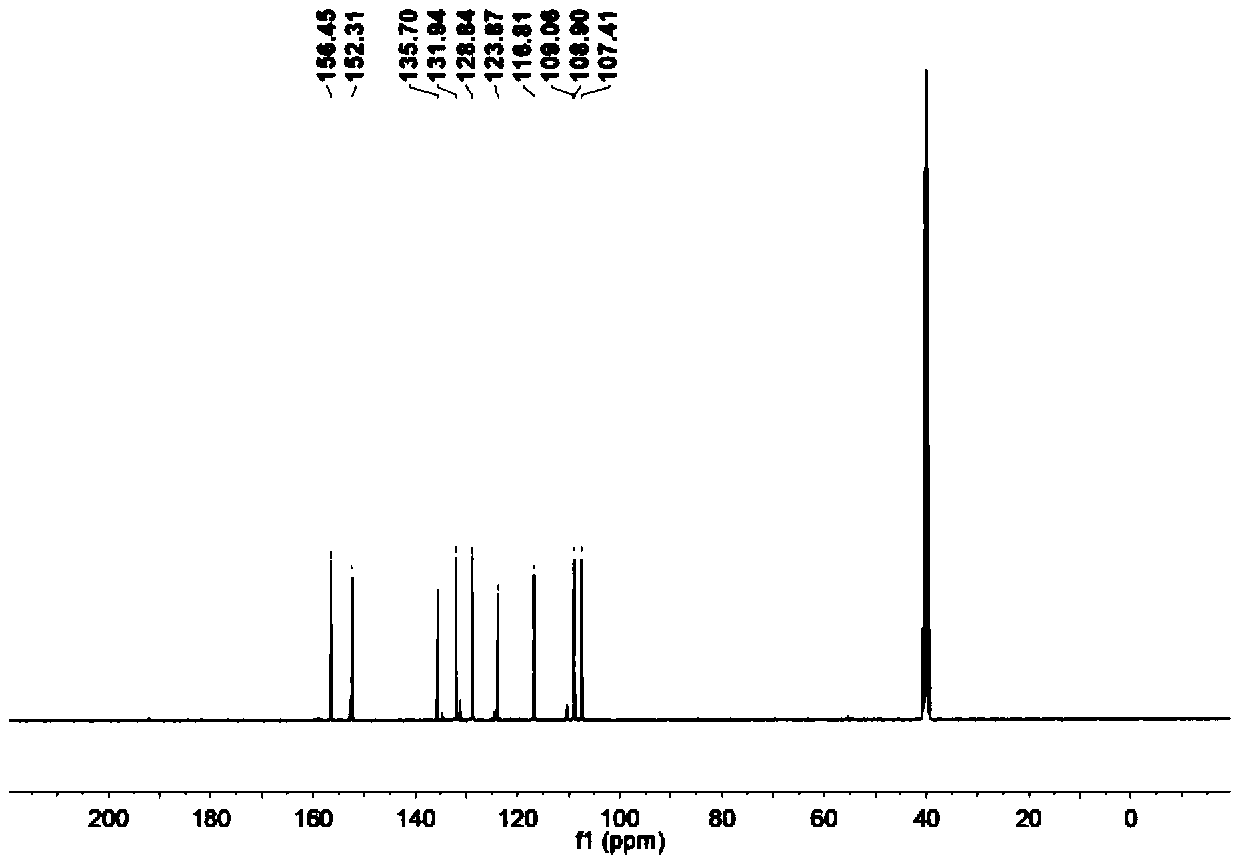
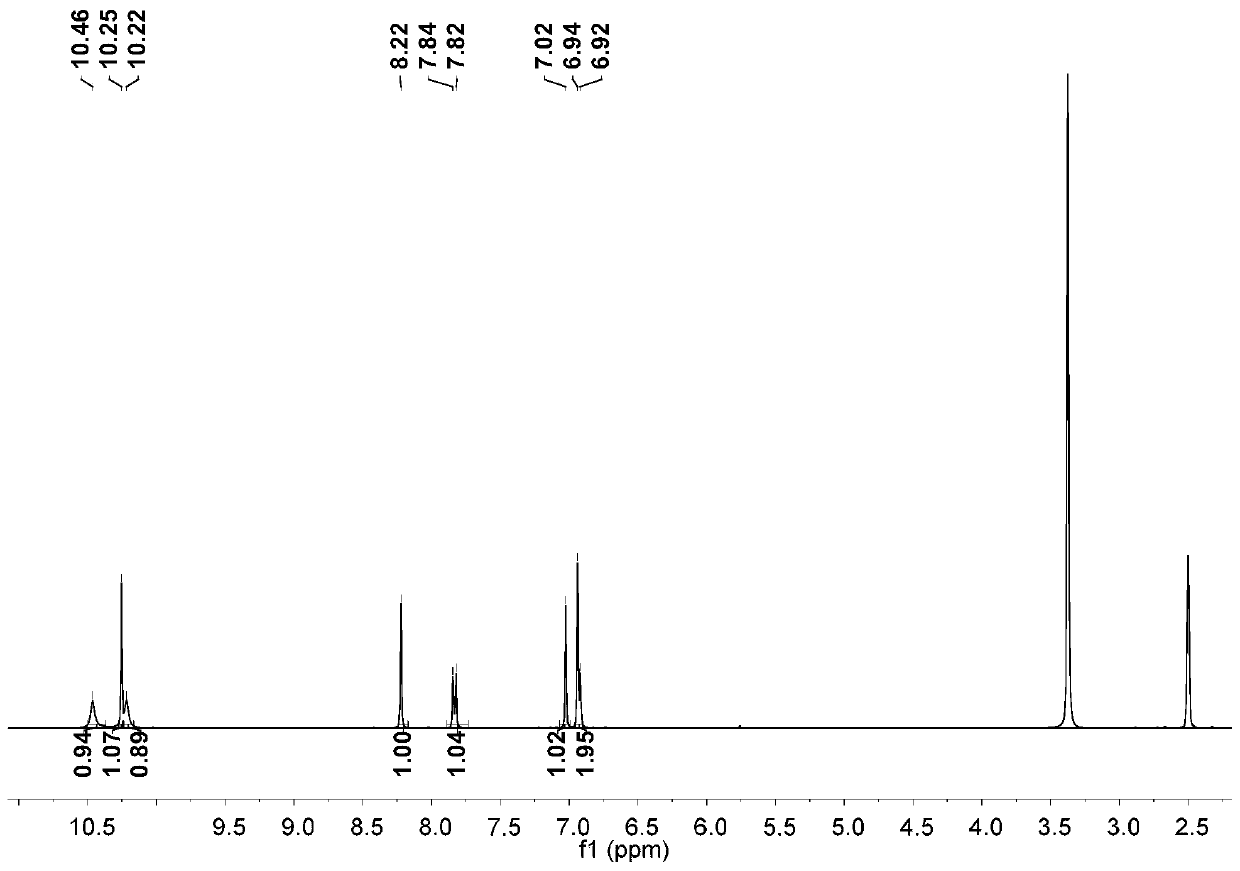
[0025] Step 1: Add glacial acetic acid to 2,7-dihydroxynaphthalene. Add liquid bromine in glacial acetic acid solution with a constant pressure funnel at 10-15°C. After dripping, react at 60°C for one hour; then add an appropriate amount of water and tin powder, adjust the temperature to 80°C, and react overnight; carry out suction filtration the next day, wash the solid with water, and finally extract with ethyl acetate to obtain crude product; after drying the organic phase, the crude product is chromatographed using an eluent and through a thin-layer column to obtain the first compound.
[0026] Step 2: Add tetrahydrofuran to the first compound, dissolve it and remove oxygen, then lower the temperature at -76°C, add tert-butyllithium, and a solid is formed; after 40 minutes of reaction, raise the reaction temperature to room temperature for reaction One hour, then cool down at -76°C for 30 minutes; then add dry N,N-dimethylformamide, gradually warm up to room temperature, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com