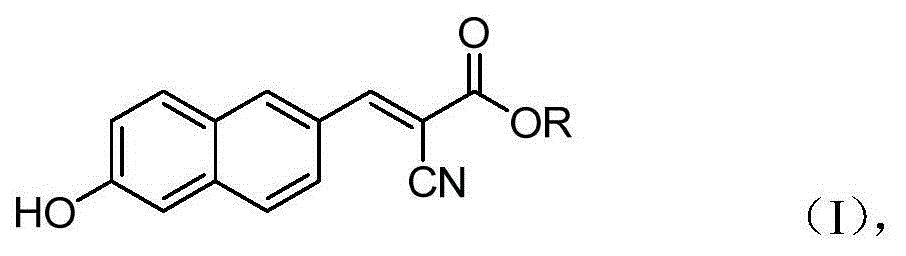
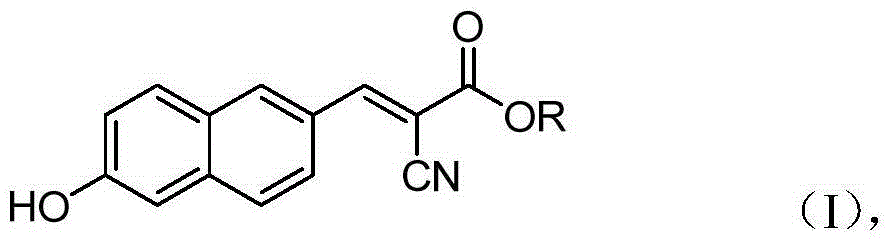
6'-hydroxyl naphthyl-2-cyanoacrylate and application thereof
A technology of cyanoacrylate and hydroxynaphthyl, which is applied in the field of acrylate compounds and 2-cyanoacrylate, and can solve the problems of undetected fluoride ion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Preparation of 2-(2-(2-methoxyethoxy)ethoxy)ethyl 6'-hydroxynaphthyl-2-cyanoacrylate
[0027] In a 100ml flask, add 3.44g 6-hydroxy-2-naphthaldehyde, 4.62g 2-(2-(2-methoxyethoxy)ethoxy) 2-cyanoacetate and 0.5g N,N-di methyl-4-aminopyridine, and then add 30 ml of anhydrous tetrahydrofuran. After reacting at room temperature for 24 hours, the solvent was distilled off under reduced pressure, and the residual solid was purified with a silica gel column under the condition that the eluent composition was ethyl acetate:n-hexane=1:1 (v / v) to obtain 7.24 g of a yellow solid. Yield 94%.
[0028] 2. Compound Characterization
[0029] Mp 137.0~138.5℃.
[0030] 1 H NMR (400MHz, DMSO-d 6 ): δ3.22(s,3H),3.37~3.44(m,4H),3.52~3.56(m,10H),3.60~3.63(m,2H),3.74~3.76(m,2H),4.40~4.43 (m,1H),7.23(s,2H),7.85~7.90(dd,J=9.16Hz,J=21.16Hz,2H),8.13~8.16(dd,J=1.39,J=8.32Hz,1H), 8.42~8.46(d,J=13.53Hz 2H),10.45(s,1H).
[0031] 13 C NMR (100MHz, DMSO-d 6 ):δ57.98,65.32,68.06,69.59,69.73,...
Embodiment 2
[0049] 1. Preparation of methyl 6'-hydroxynaphthyl-2-cyanoacrylate
[0050] Add 3.44g of 6-hydroxy-2-naphthaldehyde, 1.98g of methyl 2-cyanoacetate and 0.5g of N,N-dimethyl-4-aminopyridine into a 100ml flask, and then add 30ml of anhydrous tetrahydrofuran. After reacting at room temperature for 24 hours, the solvent was distilled off under reduced pressure, and the residual solid was purified with a silica gel column under the condition that the eluent composition was ethyl acetate:n-hexane=1:4 (v / v) to obtain 4.08 g of a yellow solid. Yield 81%.
[0051] 2. Compound Characterization
[0052] 1 H NMR (400MHz, DMSO-d 6 ): δ3.71(s,3H),4.08~4.13(m,1H),7.23(s,2H),7.81~7.88(dd,2H),8.01~8.05(dd,1H),8.40~8.43(d ,2H),10.56(s,1H).
[0053] 13 C NMR (100MHz, DMSO-d 6 ): δ52.11, 100.34, 109.11, 117.22, 121.13, 123.41, 132.50, 136.32, 137.62, 155.17, 157.55, 161.31.
[0054] The above detection results confirm that the prepared compound is 6'-hydroxynaphthyl-2-methyl cyanoacrylate...
Embodiment 3
[0061] 1. Preparation of ethyl 6'-hydroxynaphthyl-2-cyanoacrylate
[0062] Add 3.44g of 6-hydroxy-2-naphthaldehyde, 2.26g of ethyl 2-cyanoacetate and 0.5g of N,N-dimethyl-4-aminopyridine into a 100ml flask, and then add 30ml of anhydrous tetrahydrofuran. After reacting at room temperature for 24 hours, the solvent was distilled off under reduced pressure, and the residual solid was purified with a silica gel column under the condition that the eluent composition was ethyl acetate:n-hexane=1:4 (v / v) to obtain 4.65 g of a yellow solid. Yield 87%.
[0063] 2. Compound Characterization
[0064] 1 H NMR (400MHz, DMSO-d 6 ):δ1.03(s,3H),4.11~4.13(m,2H),4.55~4.58(m,1H),7.11(s,2H),7.74~7.78(dd,2H),7.89~7.92(dd ,1H), 8.10~8.14(d,2H), 10.77(s,1H).
[0065] 13 C NMR (100MHz, DMSO-d 6 ): δ11.44, 56.11, 103.11, 109.53, 116.92, 121.73, 123.81, 131.97, 136.71, 137.23, 155.51, 158.44, 162.89.
[0066] The above test results confirm that the prepared compound is 6'-hydroxynaphthyl-2-cya...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com