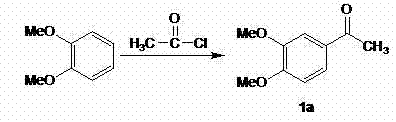Synthesis method of anti-tumor targeted therapeutic drug tivozanib
A synthesis method and a technology for targeted therapy, which are applied in the synthesis process of compounds and the synthesis route of the anti-tumor targeted therapy drug tivozanib, can solve the problems of few research reports on the synthesis process of the drug, and achieve low cost, high yield, The effect of cheap and readily available synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example one 3,4-dimethoxyacetophenone (compound 1a )Synthesis
[0034]
[0035] In a 250ml three-necked flask, add 80ml of chloroform and 22.0g (0.16mol) of anhydrous aluminum trichloride, and dropwise add 10.2g (0.13mol) of acetyl chloride and 13.8g (0.1mol) of phthalate in sequence at room temperature. After completion, stir at room temperature until the end of the reaction (GLC tracking). The above reaction solution was poured into 500ml of dilute hydrochloric acid, stirred, the organic phase was separated, the aqueous phase was extracted with chloroform, the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 15.0g of white powder compound 1a (C 10 H 12 O 3 ), m.p. 48-52°C, yield 83%. IR (cm -1 ): 1673, 1585, 1515, 1418; 1 H-NMR (CDCl 3 / ppm): δ 2.55 (s, 3H), 3.73 (s, 3H), 3.73 (s, 3H), 6.77 (s, 1H), 7.26 (s, 1H), 7.31 (s, 1H).
Embodiment 2
[0036] Example two 3,4-dimethoxy-6-nitroacetophenone (compound 1b )Synthesis
[0037]
[0038] In a 500ml three-necked bottle, add 100ml formic acid and 18g (0.1mol) of the compound 1a , 60ml of concentrated nitric acid was added dropwise below 10°C, the dropping was completed, the temperature was raised to 60-70°C, and stirred for 30min. The above reaction solution was poured into a 500ml ice-water bath, stirred and filtered to obtain 36.9g of light yellow powder compound 1b (C 10 H 11 NO 5 ), m.p. 135-137°C, 82% yield. 1 H-NMR (CDCl 3 / ppm): δ 2.50 (s, 3H), 3.97 (s, 3H), 3.99 (s, 3H), 6.76 (s, 1H), 7.62 (s, 1H).
Embodiment 3
[0039] Example three 2-amino-4,5-dimethoxyacetophenone (compound 1c )Synthesis
[0040]
[0041] In a 250ml three-necked flask, add 36ml water and 7g (0.125mol) reduced iron powder, heat up, reflux for 1h, slowly add 5.6g (0.025mol) compound 1b , stirred for 3h, suction filtered, and the filtrate was cooled to obtain 7g of yellow powder compound 1c (C 10 H 13 NO 3 ), m.p. 106-108°C, yield 96%. 1 H-NMR (CDCl 3 / ppm): δ 2.56 (s, 3H), 3.84 (s, 3H), 3.88 (s, 3H), 6.10 (s, 1H), 7.11 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com