Dicyanomethylene-4H-pyran derivatives as well as preparation method and application thereof
A technology of dicyanomethylene and pyran derivatives, applied in chemical instruments and methods, luminescent materials, color-changing fluorescent materials, etc., can solve problems affecting the luminescent properties of organic molecules, fluorescence quenching, etc., and achieve good scientific research value and industrial application potential, easy purification, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0066] Dicyano methylene-4H-pyran derivative, its structural formula is as follows:
[0067]
[0068] The preparation method of dicyano methylene-4H-pyran derivative comprises the following steps:
[0069] (1) Using 2,6-dimethyl-4-pyrone 1 as a starting material, an addition-elimination reaction occurs with malononitrile to obtain intermediate 2;
[0070] (2) Addition-elimination reaction of intermediate 2 with indole aldehydes with different alkyl chains to obtain dicyanomethylene-4H-pyran derivatives.
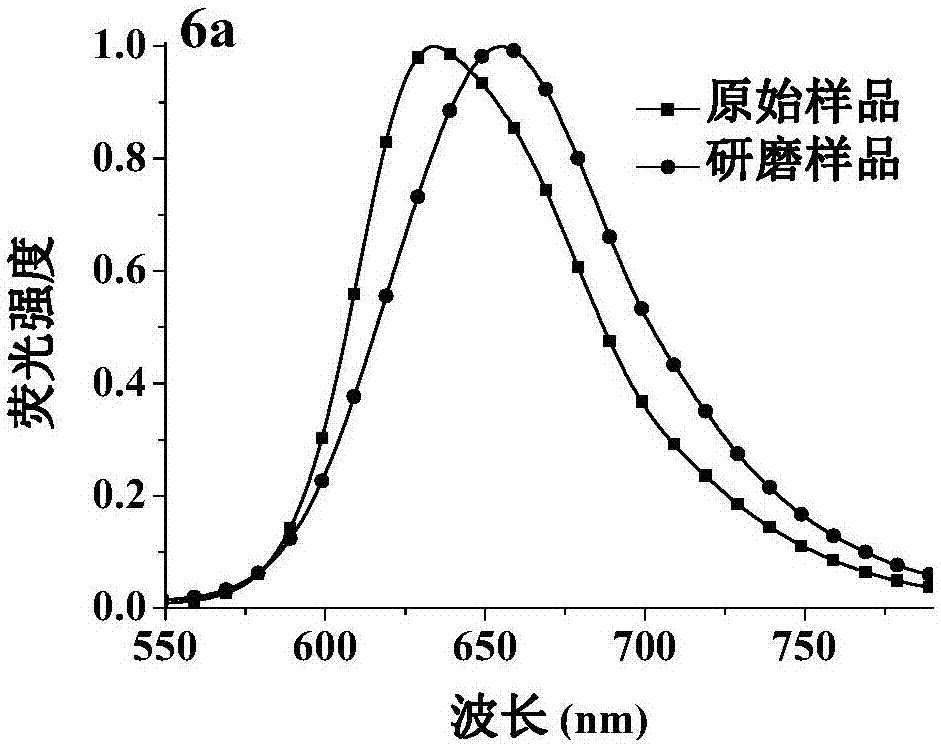
[0071] Characterization of the dicyanomethylene-4H-pyran derivative 6a: 1 H NMR (DMSO-d 6 ,500MHz), δ(ppm):11.87(s,2H),8.25(d,J=8.0Hz,2H),8.04-7.98(m,4H),7.51(d,J=8.0Hz,2H),7.28 -7.19(m,6H),6.92(s,2H). 13 C NMR (DMSO-d 6 , 125MHz), δ (ppm): 160.3, 156.3, 137.6, 132.6, 132.5, 124.6, 122.7, 120.9, 120.8, 116.6, 113.3, 112.7, 112.4, 104.3, 52.6. MS(ESI,m / z):427.10(M + +H). Anal.calcd for C 28 h 18 N 4 O: C, 78.86; H, 4.25; N, 13.14. Found: C, 79.29; H, 4.22; N, 13....
specific Embodiment 2
[0087] Dicyano methylene-4H-pyran derivative, its structural formula is as follows:
[0088]
[0089] The preparation method of dicyano methylene-4H-pyran derivative comprises the following steps:
[0090] (1) Using 2,6-dimethyl-4-pyrone 1 as a starting material, an addition-elimination reaction occurs with malononitrile to obtain intermediate 2;
[0091] (2) Addition-elimination reaction of intermediate 2 with indole aldehydes with different alkyl chains to obtain dicyanomethylene-4H-pyran derivatives.
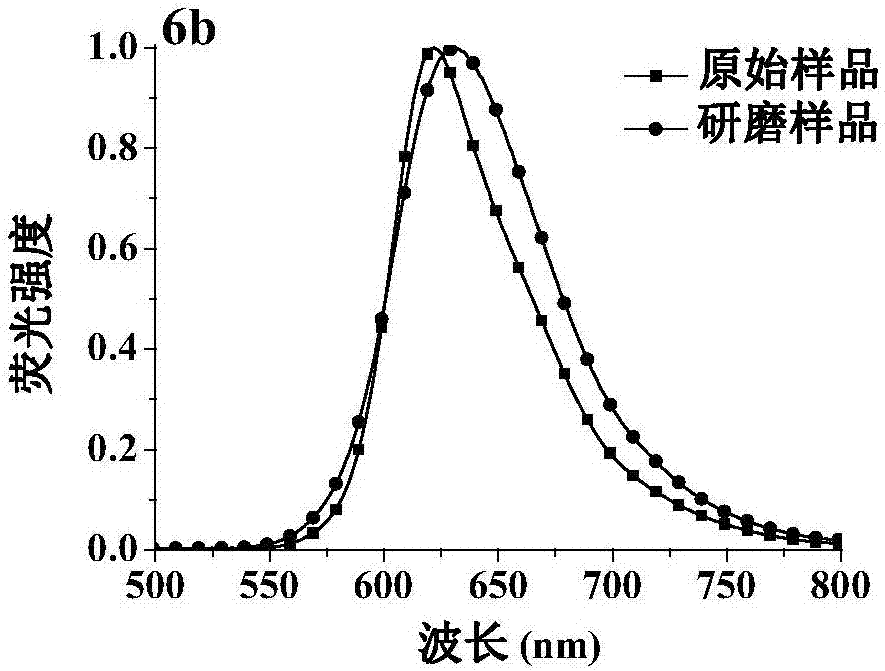
[0092] Characterization of the dicyanomethylene-4H-pyran derivative 6b: 1 H NMR (CDCl 3 ,500MHz), δ(ppm):7.94(d,J=7.5Hz,2H),7.72(d,J=15.5Hz,2H),7.51(s,2H),7.23(d,J=8.0Hz,2H ),7.36-7.30(m,4H),6.67(d,J=16.0Hz,2H),6.48(s,2H),4.19(t,J=7.0Hz,4H),1.91-1.87(m,4H) , 1.43-1.37 (m, 4H), 0.99 (t, J=7.0Hz, 6H). 13 C NMR (CDCl 3 ,125MHz), δ (ppm): 159.7, 156.2, 137.6, 132.1, 131.2, 125.9, 123.2, 121.6, 120.5, 116.5, 113.5, 112.9, 110.5, 104.8, 55.6, 46.7, 32.0, 20.2, 13.6. MS(ES...
specific Embodiment 3
[0113] Dicyano methylene-4H-pyran derivative, its structural formula is as follows:
[0114]
[0115] The preparation method of dicyano methylene-4H-pyran derivative comprises the following steps:
[0116] (1) Using 2,6-dimethyl-4-pyrone 1 as a starting material, an addition-elimination reaction occurs with malononitrile to obtain intermediate 2;
[0117] (2) Addition-elimination reaction of intermediate 2 with indole aldehydes with different alkyl chains to obtain dicyanomethylene-4H-pyran derivatives.
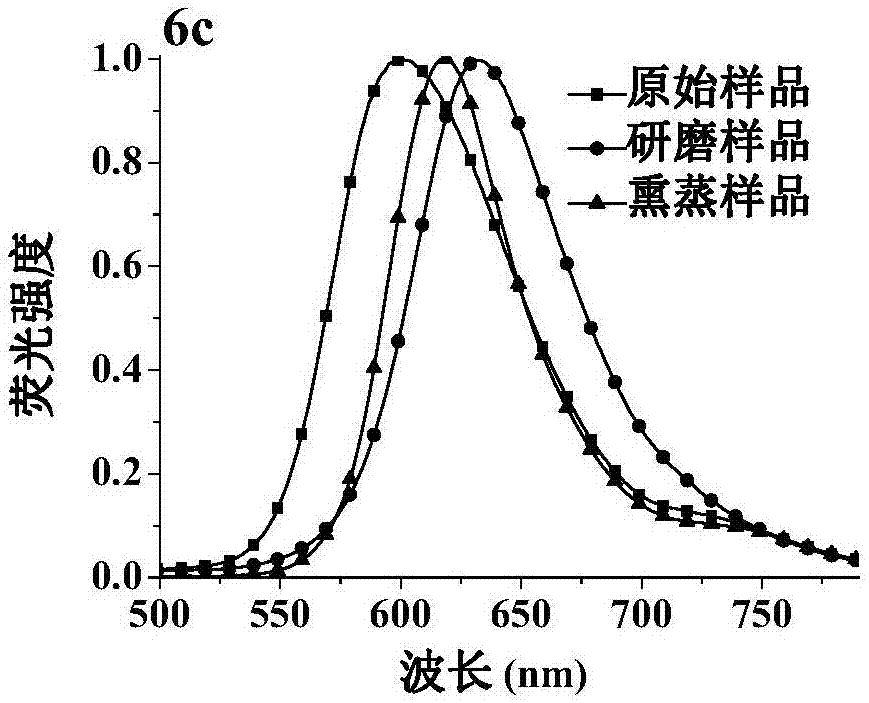
[0118] Characterization of the dicyanomethylene-4H-pyran derivative 6c: 1 H NMR (CDCl 3 ,500MHz), δ(ppm):7.96-7.94(m,2H),7.73(dd, 3 J=16.0Hz, 4 J=3.5Hz, 2H), 7.49(d, J=3.5Hz, 2H), 7.42(dd, 3 J=7.5Hz, 4 J=3.0Hz, 2H), 7.36-7.27(m, 4H), 6.69(dd, 3 J=16.0Hz, 4 J=3.5Hz, 2H), 6.49(d, J=3.5Hz, 2H), 3.39(dd, 3 J=7.0Hz, 4 J=3.5Hz, 4H), 2.29-2.24(m, 2H), 0.99(dd, 3 J=6.5Hz, 4 J=3.5Hz, 12H). 13 C NMR (CDCl 3 ,125MHz), δ (ppm): 159.7, 156.2, 137.8, 132.8, 131.3, 125.8, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com