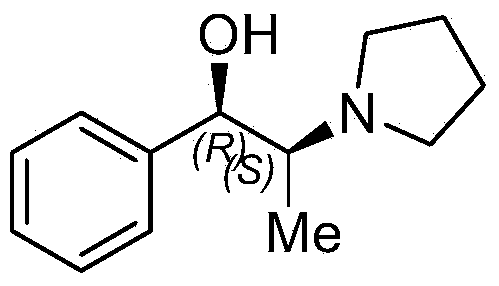
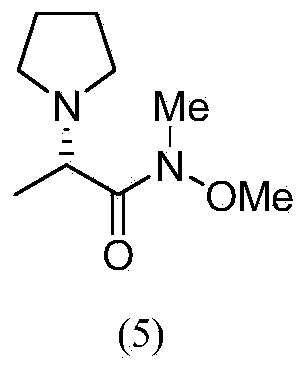
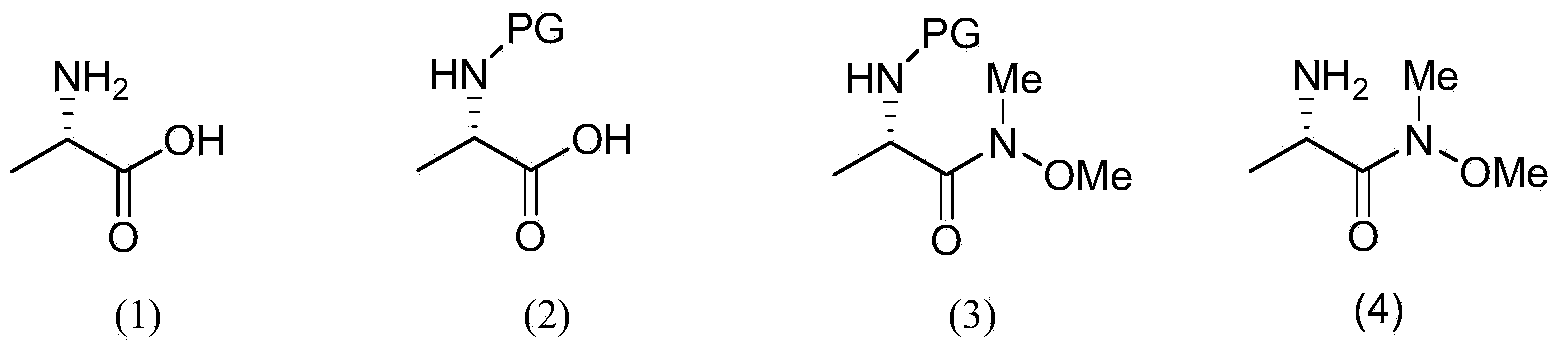
(S)-N-methoxy-methyl-2-(pyrrolidine) propionamide and preparation method and application thereof
A technology of tetrahydropyrrolyl and methoxy, which is applied in the field of synthesis of efavirenz chiral ligands, can solve the problems of difficulty in obtaining and limiting the preparation of chiral ligands, and achieve simple operation, great implementation value and Socioeconomic benefits, the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of (S)-tert-butoxycarbonylalanine
[0037] Will K 2 CO 3(30.1g, 217.8mmol) and L-alanine (10g, 112.3mmol) were dissolved in water (80mL), under nitrogen protection, a THF solution of di-tert-butyl dicarbonate (25.7g, 118mmol) was dissolved at 0°C ( 40 mL) was slowly added dropwise to the above solution to maintain the pH at 10-12. Stirring was continued at room temperature for 8 h. TLC showed that there was no remaining raw material. After the reaction was complete, the reaction was concentrated under reduced pressure to remove the solvent, and acidified with citric acid until pH = 2. Extracted with ethyl acetate (100 mL×3), dried the organic phase with anhydrous sodium sulfate, concentrated under reduced pressure to obtain 18.5 g of (S)-tert-butoxycarbonylalanine as a white solid, with a yield of 86%. Melting point: 75-77°C.
Embodiment 2
[0038] Embodiment 2: the preparation of (S)-benzyloxycarbonylalanine
[0039] Sodium hydroxide (4.48g, 112mmol) and L-alanine (5g, 56.2mmol) were dissolved in water (40mL), under nitrogen protection, benzyl chloroformate (10.3g, 60mmol) was slowly added dropwise at 0°C Into the above solution, maintain the pH at 10-12. Stirring was continued at room temperature for 6 h. TLC showed that there was no raw material remaining. The reaction was completed, and citric acid was added to adjust the pH to 2. Extracted with ethyl acetate (50 mL×3), dried the organic phase with anhydrous sodium sulfate, concentrated under reduced pressure to obtain (S)-benzyloxycarbonylalanine as a white solid 9.1 g, yield 73%. Melting point: 83-85°C.
Embodiment 3
[0040] Embodiment 3: Preparation of (S)-tert-butoxycarbonyl amino-1-methyl (methoxy) amino-propionamide
[0041] Dissolve (S)-tert-butoxycarbonylalanine (15g, 79.3mmol) in dichloromethane (200mL), slowly add carbonyldiimidazole (14.2g, 87.3mmol), and stir at room temperature for 1 hour. Then N,O-dimethylhydroxylamine hydrochloride (8.5 g, 87.3 mmol) was added, and the reaction was stirred at room temperature for 16 hours. Ethyl acetate (100mL×3) was added for extraction, and the combined organic phases were washed with 1mol / L HCl aqueous solution (20mL×2), saturated NaHCO 3 Aqueous solution (30mL×2), washed with saturated brine (40mL×2). The organic phase was dried over anhydrous sodium sulfate. Concentrate under reduced pressure and evaporate the solvent to obtain 18.3 g of (S)-tert-butoxycarbonylamino-1-methyl(methoxy)amino-propionamide as a white solid, with a yield of 99%. m.p.: 144.5°C, literature 144±5°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com