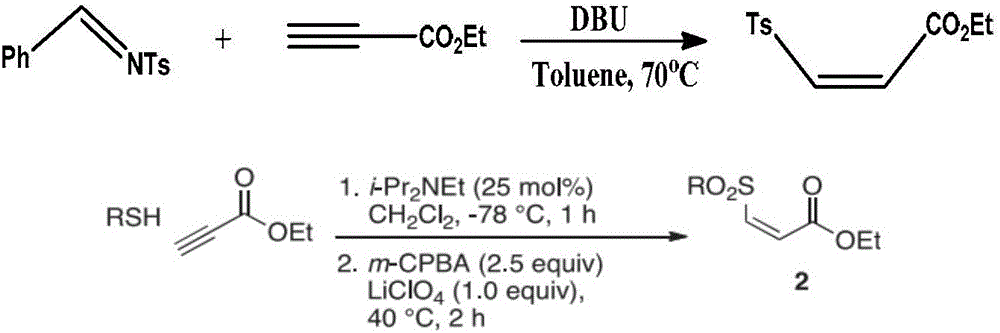
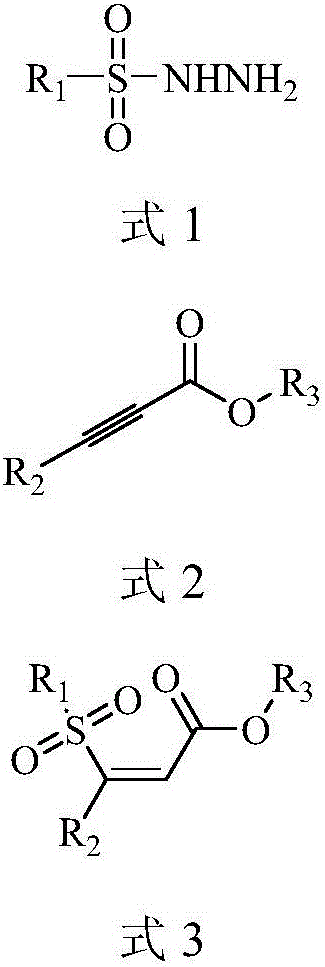
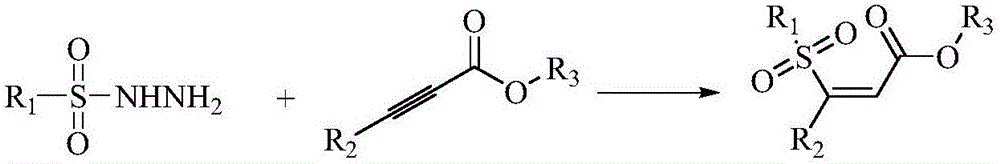
Preparation method of (Z)-sulfonyl olefine acid ester
A technology of sulfonyl enoate and alkynoate, which is applied in the field of preparation of sulfonyl enoate, can solve the problems of low selectivity, low atom economy, narrow substrate range, etc., and achieve simplified process steps, Wide application value, high selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] At room temperature, benzenesulfonylhydrazide (43mg, 0.25mmol), ethyl propiolate (74mg, 0.75mmol) and water (2mL) were successively added into a 10mL round bottom flask. Then, the reaction mixture was stirred at 70° C. for 50 minutes (reaction checked by TLC). Then, stop the reaction, add 10 mL of water, extract with dichloromethane (3*10 mL), combine the organic phases, dry over magnesium sulfate, and filter with suction. Finally wash with the mixed eluent of petroleum ether and ethyl acetate (petroleum ether and ethyl acetate volume ratio 5:1), flash column chromatography (silica gel column) obtains corresponding product (Z)-sulfonylenoate compound (Colorless liquid 52.8mg, yield 88%).
[0034] The reaction formula of this embodiment is:
[0035]
[0036] 1 H NMR (400MHz, CDCl 3 )δ7.91(d, J=8.2Hz, 2H), 7.56(s, 1H), 7.50(t, J=7.6Hz, 4H), 6.45(d, J=2.1Hz, 2H), 4.29(q, J=7.1Hz, 3H), 1.36–1.27(m, 5H); 13 C NMR (100MHz, CDCl 3 )δ163.99,139.43,135.16,134.05,131.95,1...
Embodiment 2
[0038] At room temperature, p-fluorobenzenesulfonyl hydrazide (48mg, 0.25mmol), ethyl propiolate (74mg, 0.75mmol) and water (2mL) were successively added into a 10mL round bottom flask. Then, the reaction mixture was stirred at 80° C. for 80 minutes (reaction checked by TLC). Then, stop the reaction, add 10 mL of water, extract with dichloromethane (3*10 mL), combine the organic phases, dry over magnesium sulfate, and filter with suction. Finally wash with the mixed eluent of petroleum ether and ethyl acetate (petroleum ether and ethyl acetate volume ratio 5:1), flash column chromatography (silica gel column) obtains corresponding product (Z)-sulfonylenoate compound (yellow solid 58.1 mg, yield 90%).
[0039] The reaction formula of this embodiment is:
[0040]
[0041] 1 H NMR (400MHz, CDCl 3 )δ8.06(dd, J=8.8,5.1Hz,2H),7.22(d,J=8.1Hz,2H),6.56(s,2H),4.40(q,J=7.1Hz,2H),1.42( t,J=7.1Hz,3H); 13 C NMR (100MHz, CDCl 3 )δ167.35, 164.79, 163.89, 135.53, 135.18, 132.19, 131....
Embodiment 3
[0043] At room temperature, p-chlorobenzenesulfonyl hydrazide (52mg, 0.25mmol), ethyl propiolate (74mg, 0.75mmol) and water (2mL) were successively added into a 10mL round bottom flask. The reaction mixture was then stirred at 55°C for 80 minutes (reaction checked by TLC). Then, stop the reaction, add 10 mL of water, extract with dichloromethane (3*10 mL), combine the organic phases, dry over magnesium sulfate, and filter with suction. Finally wash with the mixed eluent of petroleum ether and ethyl acetate (petroleum ether and ethyl acetate volume ratio 5:1), flash column chromatography (silica gel column) obtains corresponding product (Z)-sulfonylenoate compound (White solid 62.6 mg, yield 91%).
[0044] The reaction formula of this embodiment is:
[0045]
[0046] 1 H NMR (400MHz, CDCl 3 )δ7.94(d, J=8.6Hz, 2H), 7.56(d,, J=8.5Hz, 2H), 6.53(s, 2H), 4.36(q, J=7.3Hz, 1H), 1.36(t ,J=7.2Hz,2H); 13 C NMR (100MHz, CDCl 3 )δ163.81, 140.91, 137.96, 135.04, 132.51, 129.79, 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com