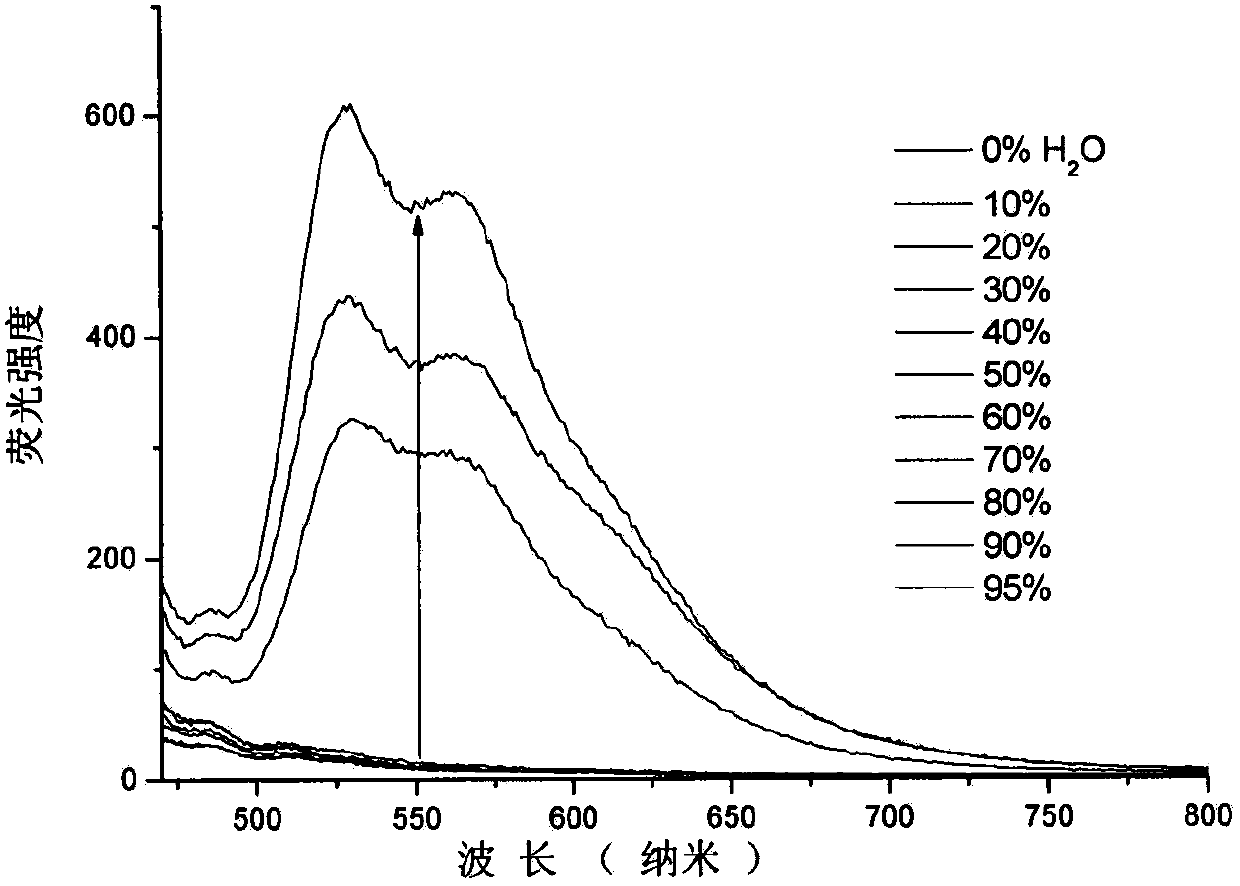
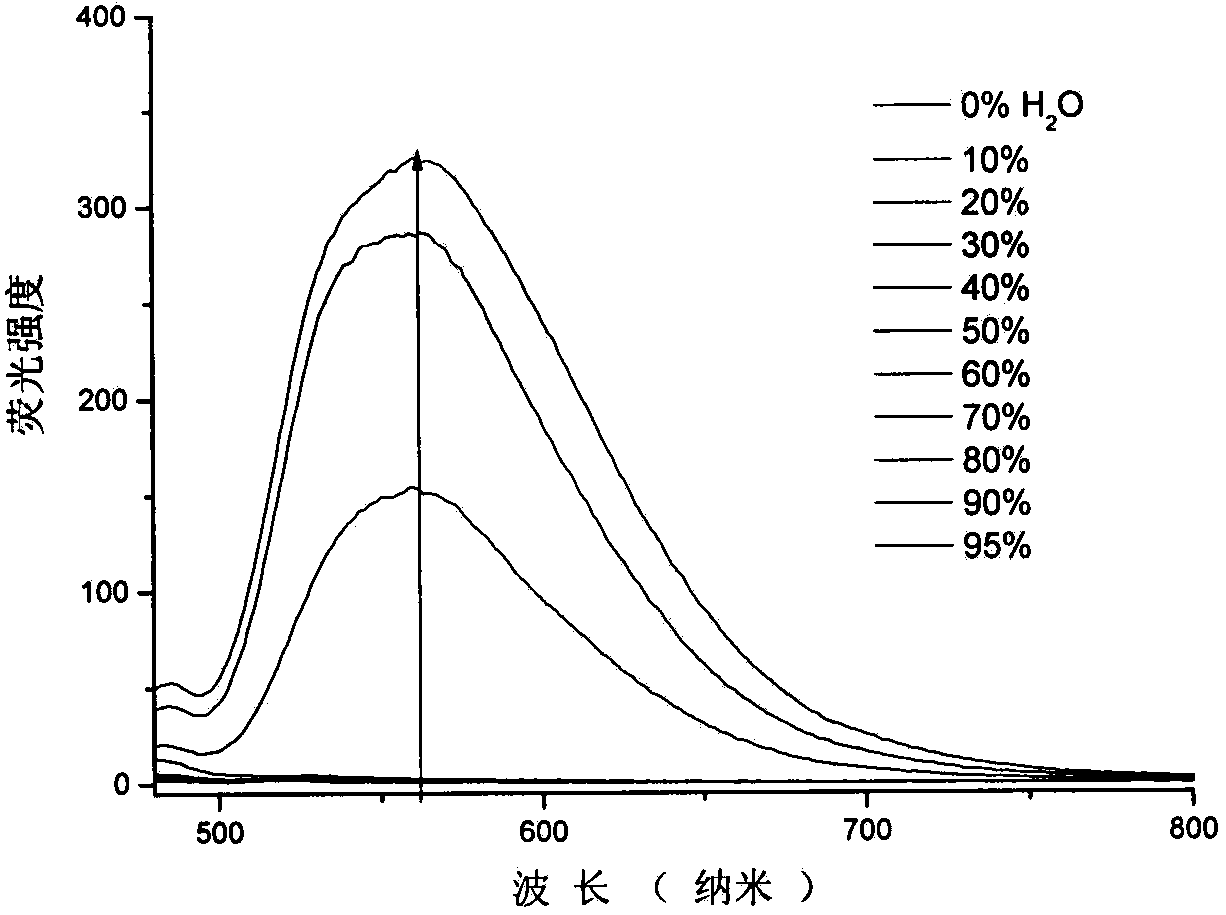
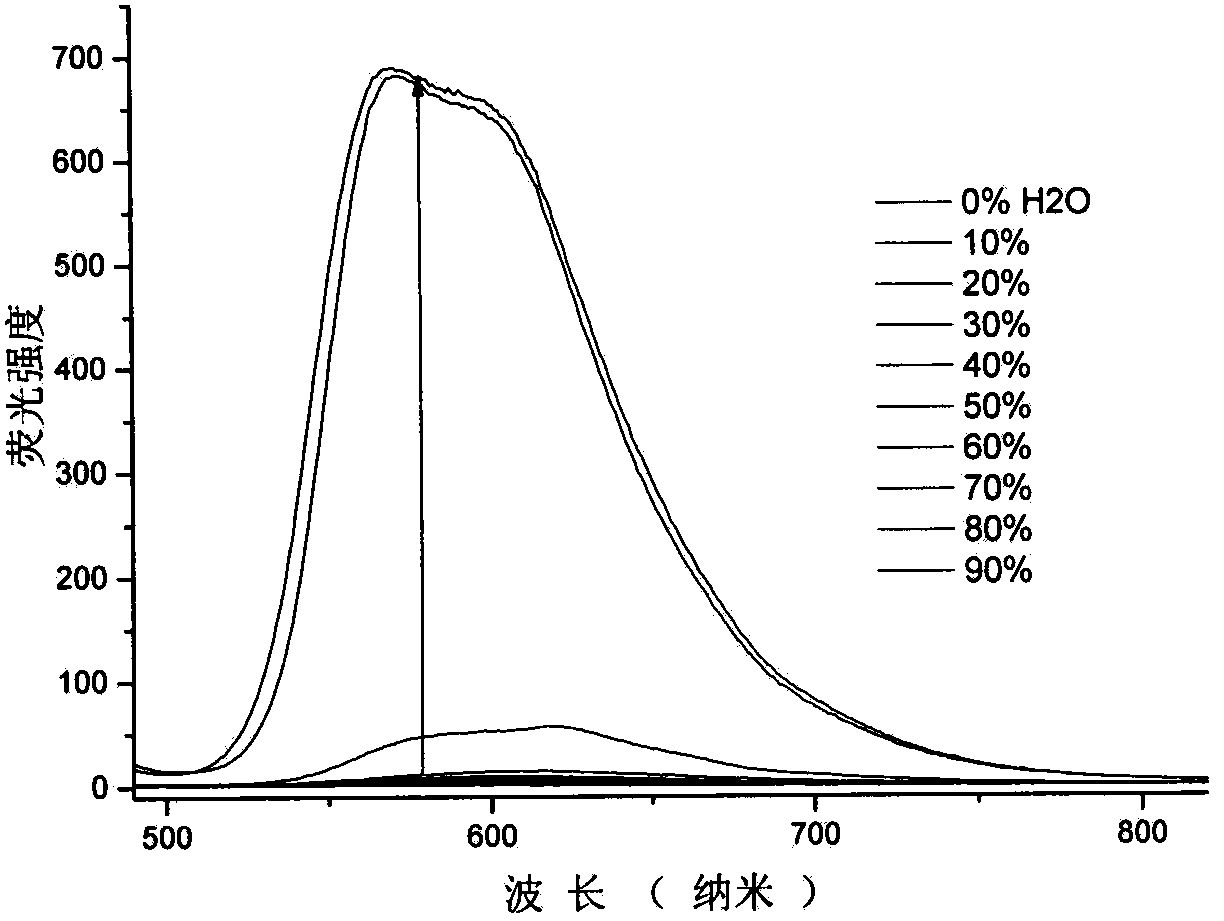
Quinoline nitrile derivative with aggregation-induced emission performance
A derivative, quinoline nitrile technology, applied in the field of aggregation-induced luminescent compounds, can solve the problems of difficult synthetic routes, fluorescence quenching, etc., and achieve the effect of less synthesis steps, simple synthesis, and obvious aggregation-induced luminescence characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] R in the compound shown in formula I below 2 Taking phenyl or substituted phenyl as an example, the method for the compound shown in the synthetic formula I is further described, and the synthetic method includes the following steps:
[0025] a) with acetonitrile as the reaction solvent, 2-methylquinoline and the corresponding haloalkane (R 1 X, X is Br or I, etc.) kept at reflux for 30 minutes to 12 hours to obtain 2-methyl-quinolinate (compound shown in formula III) substituted on the corresponding N;
[0026] b) In the ethanol / acetic acid system, the compound shown in formula III and malononitrile were kept in a reflux state for 20 hours to 36 hours, and the Michael addition and elimination reaction obtained substituted 2-methyl-quinoline nitrile (formula IV compounds shown);
[0027] c) with acetonitrile as a solvent and piperidine as a catalyst, the compound shown in formula IV and the corresponding benzaldehyde or substituted benzaldehyde are kept in a reflux st...
Embodiment 1
[0034] Synthesis of EP (compound shown in formula I-1)
[0035]
[0036] (1) Synthesis of N-ethyl-2-methylquinoline bromide
[0037] In a 100ml round bottom flask, 2-methylquinoline (7.15g, 50mmol), bromoethane (38.2g, 350mmol) and acetonitrile (50ml) were successively added, and the mixture was refluxed for 2h under Ar protection and magnetic stirrer stirring. After standing and cooling to room temperature, a yellow solid precipitated out, and the solid filter cake was collected by suction filtration to obtain 4.45 g of a yellow solid product, with a yield of 35.3%.
[0038] (2) N-ethyl-2-methyl-4-(α,α-dicyano)methylene-1,4-dihydroquinoline
[0039] In a 100ml round bottom flask, add N-ethyl-2-methylquinoline bromide (4.6g, 18.4mmol), malononitrile (3.04g, 46mmol) and absolute ethanol (35ml) successively, under argon protection , After reflux reaction for 2 hours, the filter cake was obtained by suction filtration as 3.0 g of a yellow product, and the yield was 69.4%.
...
Embodiment 2
[0046] Synthesis of EF (compound shown in formula I-2)
[0047]
[0048] (1) Synthesis of N-ethyl-2-methylquinoline bromide
[0049] In a 100ml round bottom flask, 2-methylquinoline (7.15g, 50mmol), bromoethane (38.2g, 350mmol) and acetonitrile (50ml) were successively added, and the mixture was refluxed for 2h under Ar protection and magnetic stirrer stirring. After standing and cooling to room temperature, a yellow solid precipitated out, and the solid filter cake was collected by suction filtration to obtain 4.45 g of a yellow solid product, with a yield of 35.3%.
[0050] (2) N-ethyl-2-methyl-4-(α,α-dicyano)methylene-1,4-dihydroquinoline
[0051] In a 100ml round bottom flask, add N-ethyl-2-methylquinoline bromide (4.6g, 18.4mmol), malononitrile (3.04g, 46mmol) and absolute ethanol (35ml) successively, under argon protection , After reflux reaction for 2 hours, the filter cake was obtained by suction filtration as 3.0 g of a yellow product, and the yield was 69.4%.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com