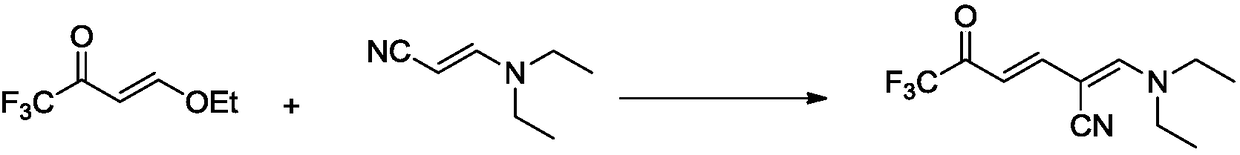
Synthetic method for 6-trifluoromethyl-nicotinic acid
A technology of trifluoromethylnicotinic acid and trifluoromethylnicotinonitrile, which is applied in the field of synthesis of 6-trifluoromethylnicotinic acid, and can solve problems such as inconvenient operation, low yield, and low process yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 376g (5.2mol) of vinyl ethyl ether, 826g of pyridine and 5000mL of dichloromethane in sequence in a 10L three-necked flask, stir well, then add 1084g (5.2mol) of phosphorus pentachloride, and pass through the constant pressure funnel at 0°C Slowly add 460g (4mol) of trifluoroacetic acid dropwise, and then stir and react at room temperature for 20h; after the reaction, the reaction system is washed once with 2000mL of water and 1000mL of 10% hydrochloric acid, and then washed with 1500mL of saturated sodium bicarbonate solution until the reaction solution The aqueous phase is neutral, and then dried over anhydrous magnesium sulfate and concentrated by suction to obtain the crude product, which is recrystallized in 2000 mL of a mixed solvent of acetone and water (V acetone: V water = 3:1) to obtain 4-ethoxy Pure 1,1,1-trifluoro-3-buten-2-one 542.0g; 1 H NMR (400MHz, CDCl 3 )δ7.89(d, J=12.3Hz, 1H), 5.85(d, J=12.3Hz, 1H), 4.10(q, J=7.1Hz, 2H), 1.40(t, J=7.1Hz, 3H) . ...
Embodiment 2
[0019] Add 376g (5.2mol) of vinyl ethyl ether, 826g of pyridine and 5000mL of dichloromethane in sequence into a 10L three-necked flask, stir well, then add 712g (5.2mol) of phosphorus trichloride, and pass through the constant pressure funnel at 0°C Slowly add 460g (4mol) of trifluoroacetic acid dropwise, and then stir and react at room temperature for 20h; after the reaction, the reaction system is washed once with 2000mL of water and 1000mL of 10% hydrochloric acid, and then washed with 1500mL of saturated sodium bicarbonate solution until the reaction solution The aqueous phase is neutral, and then dried over anhydrous magnesium sulfate and concentrated by suction to obtain the crude product, which is recrystallized in 2000 mL of a mixed solvent of acetone and water (V acetone: V water = 3:1) to obtain 4-ethoxy 536.0 g of pure 1,1,1-trifluoro-3-buten-2-one.
Embodiment 3
[0021] Add 376g (5.2mol) of vinyl ether, 826g of pyridine and 5000mL of dichloromethane in sequence into a 10L three-necked flask, stir evenly, and then add 200g of silicotungstic acid / silica gel (msilicotungstic acid: msilica gel=1:3), 460g (4mol) of trifluoroacetic acid was slowly added dropwise through a constant pressure funnel at 0°C, and after the dropwise addition was completed, the reaction was stirred at room temperature for 20h; Wash with 1500 mL of saturated sodium bicarbonate solution until the aqueous phase of the reaction solution is neutral, and then concentrate with suction after drying over anhydrous magnesium sulfate to obtain the crude product. 1) Recrystallize in 2000mL to obtain 660.0g of pure 4-ethoxy-1,1,1-trifluoro-3-buten-2-one.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com