Method for synthesizing pyridine propiolic acid ester
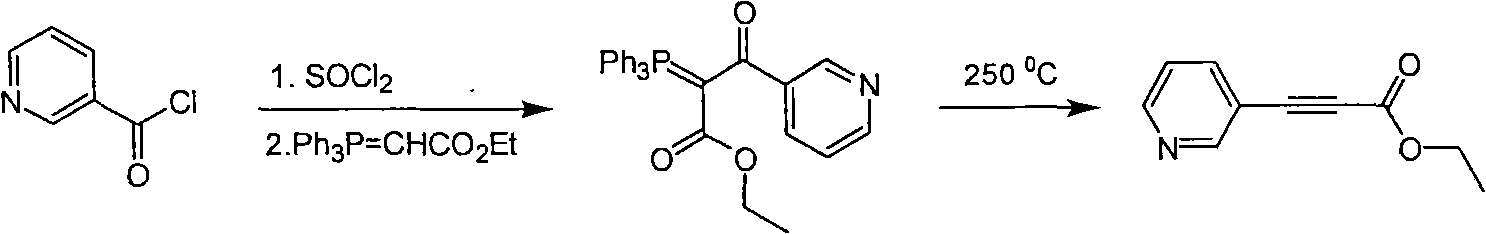
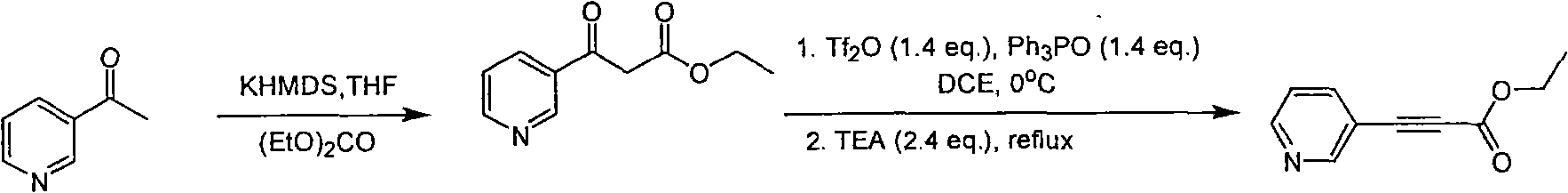
A kind of technology of pyridine propiolate and synthesis method, which is applied in the field of pyridine propiolate synthesis, can solve problems such as needing high temperature, and achieves the effect of selecting a reasonable reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
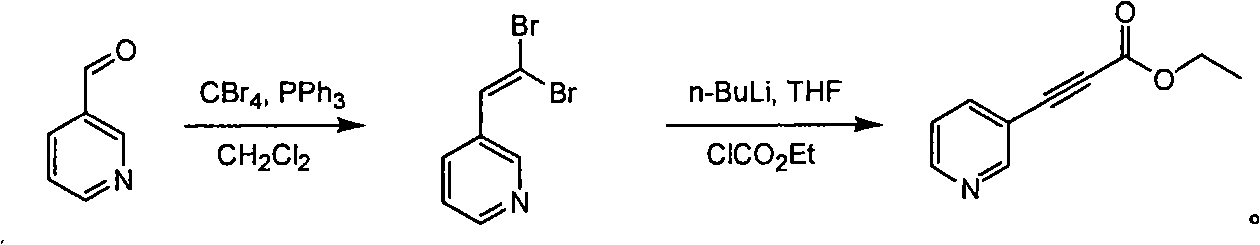
[0021] A dichloromethane solution (500 mL) dissolved with triphenylphosphine (661.0 g, 2.52 mol) was added dropwise to a dichloromethane solution (1000 mL) containing carbon tetrabromide (413 g, 1.26 mol) at 0° C., and the The mixed solution was stirred for 1 hour at 0°C. 2-Pyridinecarbaldehyde (90 g, 0.84 mol) was added dropwise to the reaction solution, and the reaction was continued at 0° C. for 1 hour. TLC (petroleum ether:ethyl acetate volume ratio=10:1) detection showed that the reaction was complete. Water (500ml) was added dropwise. Separate the organic phase and wash with brine. Anhydrous Na 2 SO 4 Drying and concentration gave crude 2-(2,2-dibromo-vinyl)-pyridine (157 g, 68.16%) as a tan oil.
[0022] At -78°C, a THF solution (500 mL) of n-BuLi (2.5 mol / L, 1.255 mol) was slowly added dropwise to crude 2-(2,2-dibromo-vinyl)-pyridine (157 g, 0.6 mol) . Stirring of the reaction was continued at -78°C for 1 hour. Ethyl chloroformate (94.74 g, 0.837 mol...
Embodiment 2
[0024]
[0025] A dichloromethane solution (600 mL) dissolved with triphenylphosphine (733.71 g, 2.80 mol) was added dropwise to a dichloromethane solution (1000 mL) containing carbon tetrabromide (458.43 g, 1.40 mol) at 0° C. The mixed solution was stirred for 1 hour at 0°C. 3-Pyridinecarbaldehyde (100 g, 0.93 mol) was added dropwise to the reaction solution, and the reaction was continued at 0° C. for 1 hour. TLC (petroleum ether:ethyl acetate volume ratio=10:1) detection showed that the reaction was complete. Water (800ml) was added dropwise. Separate the organic phase and wash with brine. Anhydrous Na 2 SO 4 Drying and concentration afforded crude 3-(2,2-dibromo-vinyl)-pyridine (207.61 g, 81.2%) as a tan oil.
[0026] At -78°C, a THF solution (664 mL) of n-BuLi (2.5 mol / L, 1.659 mol) was slowly added dropwise to crude 3-(2,2-dibromo-vinyl)-pyridine (207 g, 0.79 mol) . After continuing to stir the reaction solution at -78°C for 1 hour, ethyl chloroformate (120.02 g...
Embodiment 3
[0028]
[0029] A dichloromethane solution (600 mL) dissolved with triphenylphosphine (733.71 g, 2.80 mol) was added dropwise to a dichloromethane solution (1000 mL) containing carbon tetrabromide (458.43 g, 1.40 mol) at 0° C. The mixed solution was stirred for 1 hour at 0°C. 4-Pyridinecarbaldehyde (100 g, 0.93 mol) was added dropwise to the reaction solution, and the reaction was continued at 0° C. for 1 hour. TLC (petroleum ether:ethyl acetate volume ratio=10:1) detection showed that the reaction was complete. Water (800ml) was added dropwise. The organic phase was separated and washed with brine, anhydrous Na 2 SO 4 Drying and concentration afforded crude 4-(2,2-dibromo-vinyl)-pyridine (115.05 g, 44.5%) as a black oil.
[0030] At -78°C, a THF solution (370 mL) of n-BuLi (2.5 mol / L, 0.924 mol) was slowly added dropwise to crude 4-(2,2-dibromo-vinyl)-pyridine (115 g, 0.44 mol) . The reaction solution was stirred at -78°C for 1 hour, and ethyl chloroformate (66.84 g, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com