Near-infrared fluorescent probe and preparation method and application thereof
A fluorescent probe and near-infrared technology, applied in the field of fluorescent materials, can solve the problem of inability to achieve synergistic tumor therapy, and achieve the effect of enhancing photothermal conversion effect, promoting therapeutic effect, and improving uniform distribution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
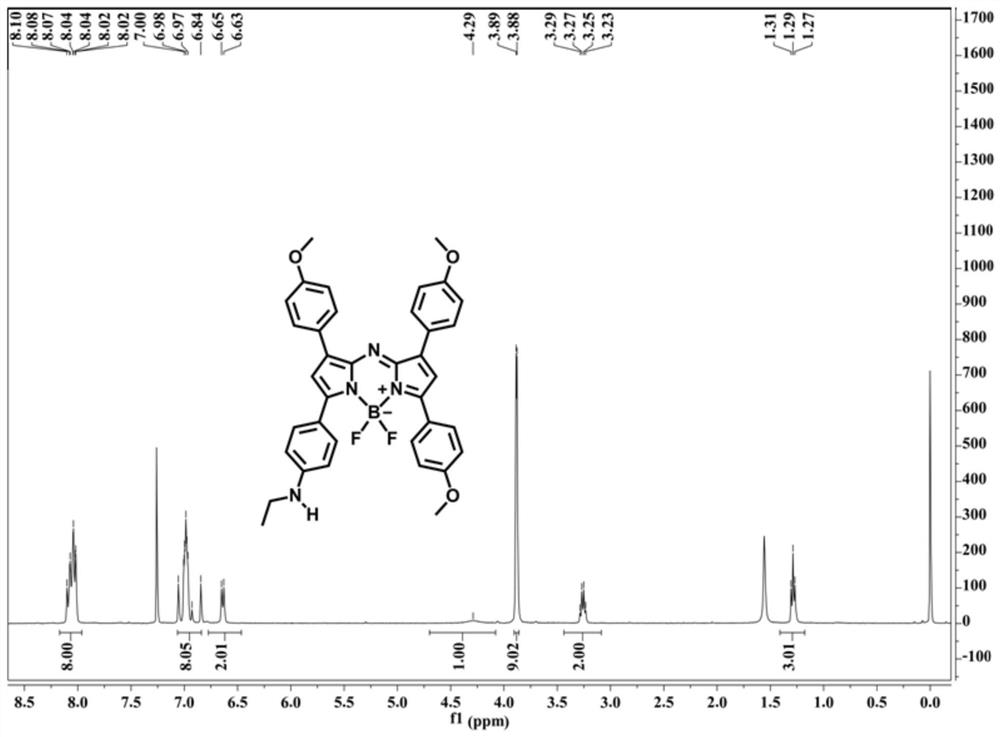
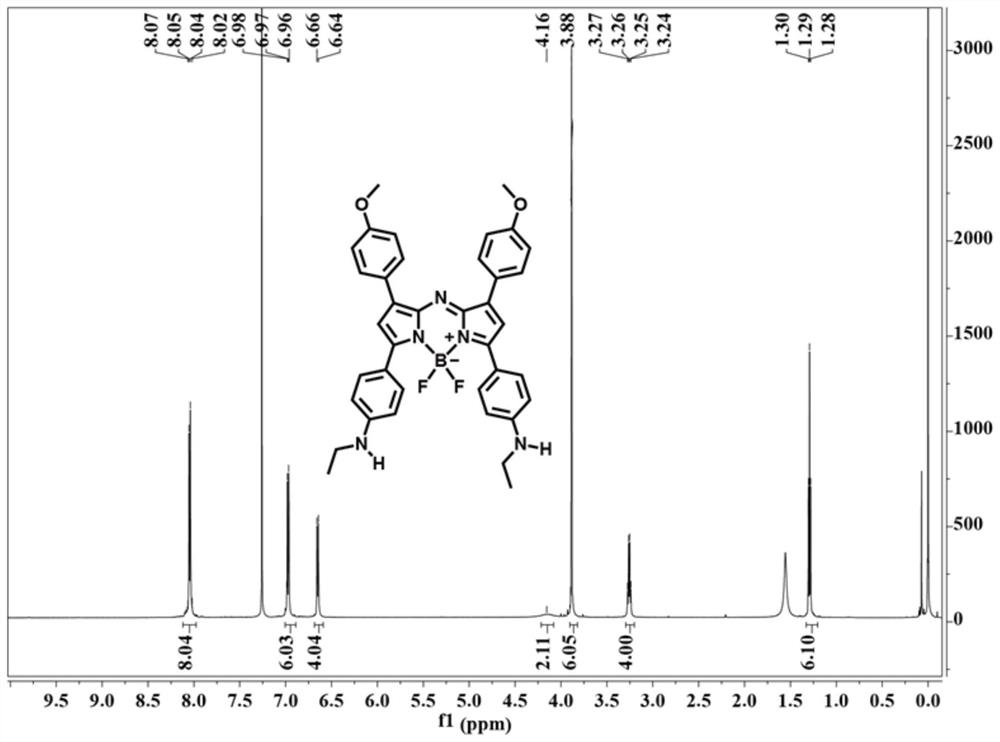
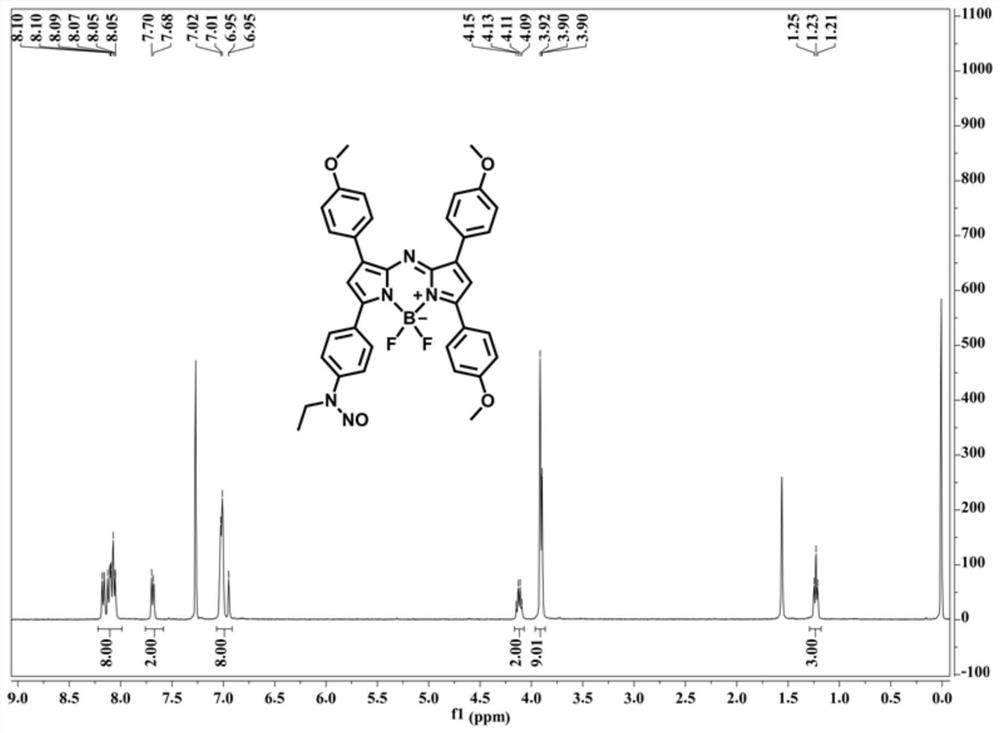
[0061] This embodiment provides the preparation method of near-infrared fluorescent probe (compound A and compound A-1), and the synthesis route of compound A and compound A-1 is as follows:
[0062]
[0063] The synthetic route of compound 4' and compound 4-1' is as follows:
[0064]
[0065] The preparation method of compound A and compound A-1 specifically comprises the following steps:
[0066] (1) Synthesis of compound 3'
[0067] In the mixed solution of 2.68g p-methoxybenzaldehyde (20mmol, compound 2'), 3.26g p-ethylaminoacetophenone (20mmol, compound 1') and 30mL absolute ethanol, slowly add 10mL sodium hydroxide solution (10wt%), reacted at room temperature for 12 hours, and filtered to obtain a yellow solid, washed with cold absolute ethanol, and dried in vacuo to obtain 3.56 g of compound 3' (63% yield).
[0068] 1 H NMR (400MHz, CDCl 3 )δ (ppm) = 7.95 (d, J = 8.4Hz, 2H), 7.75 (d, J = 15.2Hz, 1H), 7.58 (d, J = 8.4Hz, 2H), 7.45 (d, J = 15.6Hz ,1H),6.91(d,J...
experiment example 1
[0091] Adopt the following methods to detect the absorption spectra of compound 6', compound 6-1', compound A, compound A-1 respectively:
[0092] Test compound in 2mL DMSO solution (10 -6 Absorption spectra in M).
[0093] The absorption spectra of compound 6' and compound A are as Figure 5 As shown, it can be seen that the spectrum of compound 6' is red-shifted compared with that of compound A, wherein the maximum absorption wavelength of compound 6' is 768nm, and the maximum absorption wavelength of compound A is 703nm; compound 6-1' and compound A- The absorption spectrum of 1 is as Image 6 As shown, it can be seen that the spectrum of compound 6-1' is red-shifted compared with that of compound A-1, wherein the maximum absorption wavelength of compound 6-1' is 820nm, and the maximum absorption wavelength of compound A-1 is 703nm. It can be seen that compound A and compound A-1 can be used as near-infrared fluorescent probes, and can be used to prepare contrast agents ...
experiment example 2
[0095] The following methods were used to investigate the NO production of compound A and compound A-1 before and after near-infrared light irradiation:
[0096] DAF-FM DA (NO fluorescent probe) exhibits weak fluorescence, and it exhibits strong fluorescence emission after being combined with NO. 700nm laser irradiation compound A (or A-1) (10 -6 M) and DAF-FM (5 mM) in DMSO solution for 5 min, and use a fluorescence spectrometer to test the emission spectra of the mixed solution before and after irradiation.
[0097] The fluorescence spectra of the NO probe of compound A before and after near-infrared light irradiation are as follows: Figure 7 As shown, the fluorescence spectra of the NO probe of compound A-1 before and after near-infrared light irradiation are as follows: Figure 8 shown. Depend on Figure 7 and Figure 8 It can be seen that near-infrared light irradiation can also promote compound A and compound A-1 to release NO gas, and the amount of NO gas released...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com