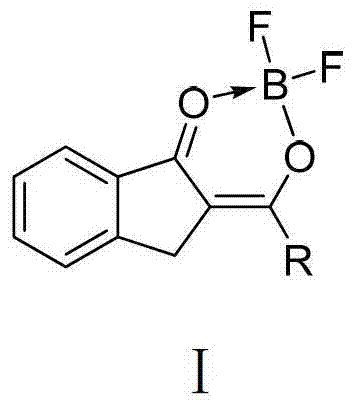
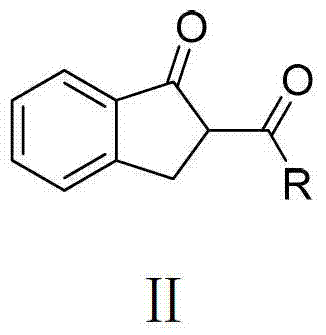
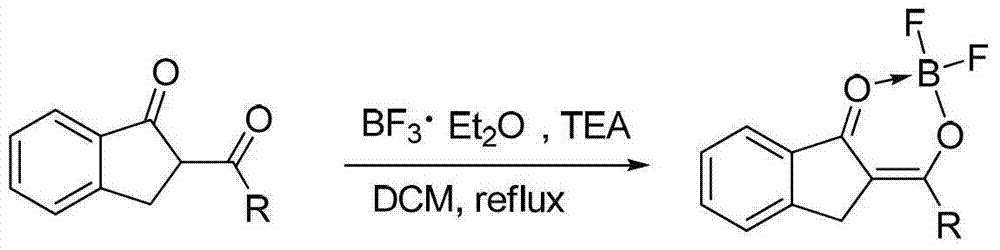
O,O two-tooth type organic boron difluoride fluorescent dye and preparation method thereof
A technology of fluorescent dyes and boron difluoride, which is applied in the field of organic boron difluoride fluorescent dyes and its preparation, to achieve the effects of high product yield, easy availability of raw materials, and large molar extinction coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The first O, O bidentate type organic boron difluoride fluorescent dye, this kind of fluorescent dye is represented as compound 1 in this embodiment 1, and the structural formula of compound 1 is:
[0037]
[0038] The method for preparing the compound 1 is as follows: put 2-benzoylindanone (5 mmol, 1.18 g) into a 50 mL two-neck flask at room temperature, and add anhydrous dichloromethane (5 mL) solution. After nitrogen purging for 2 minutes, triethylamine (30mmol, 5mL) and boron trifluoride ether (35mmol, 5mL) were injected into the reaction solution with a 10mL syringe. After reacting for 2 h at the reflux temperature of dichloromethane, the reaction was quenched with water. Extract with dichloromethane 2 to 3 times, and combine the organic phases. anhydrous Mg 2 SO 4 The organic phase is dried. Finally, after the organic phase was spin-dried in vacuum, the obtained crude product was separated and purified by a chromatographic column of 30 times its weight in s...
Embodiment 2
[0048] The second O, O bidentate organic boron difluoride fluorescent dye, this kind of fluorescent dye is represented as compound 2 in this embodiment, and the structural formula of compound 2 is:
[0049]
[0050] The method for preparing the compound 2 is as follows: at room temperature, put 2-thienoylindanone (5mmol, 1.21g) into a 50mL two-neck flask, and add anhydrous dichloromethane (5mL) solution. After nitrogen purging for 2 minutes, triethylamine (30mmol, 5mL) and boron trifluoride ether (35mmol, 5mL) were injected into the reaction solution with a 10mL syringe. After reacting for 2 h at the reflux temperature of dichloromethane, the reaction was quenched with water. Extract with dichloromethane 2 to 3 times, and combine the organic phases. anhydrous Mg 2 SO 4 The organic phase is dried. Finally, after the organic phase was spin-dried in vacuum, the obtained crude product was separated and purified by a chromatographic column of 30 times its weight in silica ge...
Embodiment 3
[0060] The third O, O bidentate type organic boron difluoride fluorescent dye, this kind of fluorescent dye is represented as compound 3 in this embodiment, and the structural formula of compound 3 is:
[0061]
[0062] The method for preparing the compound 3 is as follows: at room temperature, put 2-naphthoylindanone (5 mmol, 1.43 g) into a 50 mL two-neck flask, and add anhydrous dichloromethane (5 mL) solution. After nitrogen purging for 2 minutes, triethylamine (30mmol, 5mL) and boron trifluoride ether (35mmol, 5mL) were injected into the reaction solution with a 10mL syringe. After reacting for 2 h at the reflux temperature of dichloromethane, the reaction was quenched with water. Extract with dichloromethane 2 to 3 times, and combine the organic phases. anhydrous Na 2 SO 4 The organic phase is dried. Finally, after the organic phase was spin-dried in vacuum, the obtained crude product was separated and purified by a chromatographic column of 30 times its weight in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com