Fluorescent probe for detecting biologic thiol and preparation method and usage method thereof
A fluorescent probe and sulfhydryl technology, applied in the field of bioengineering, can solve problems such as low signal-to-noise ratio, rare probes, poor photostability, etc., and achieve the effect of single recognition, excellent selectivity, and high sulfhydryl detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
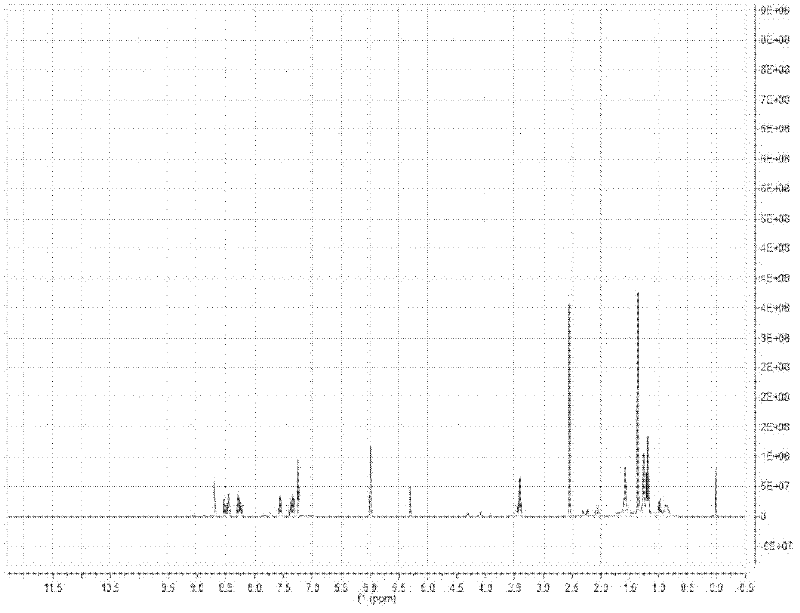
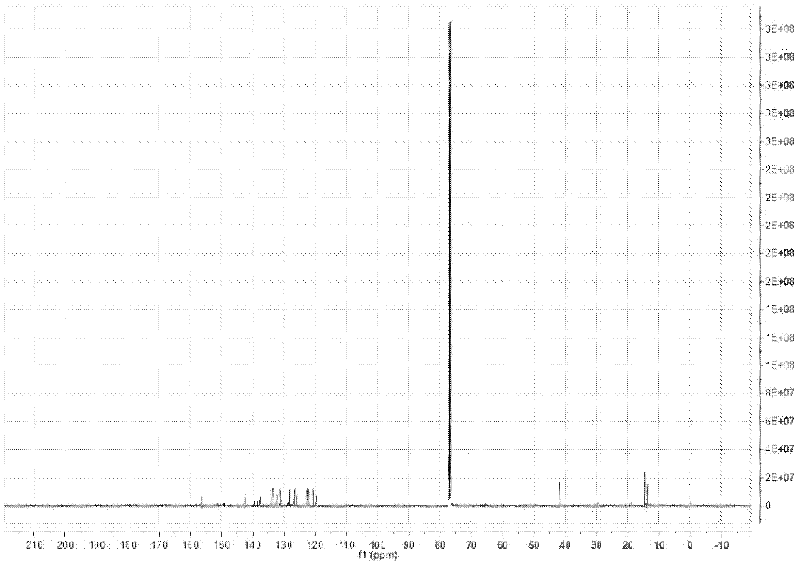
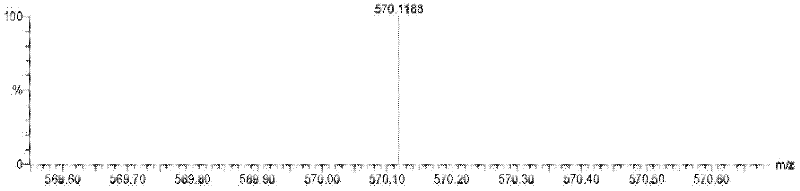
Image
Examples
Embodiment 1
[0039] (1) Preparation of probe molecules
[0040] The reaction process is represented by the following reaction scheme:
[0041]
[0042] 1) Preparation of formula II: under the protection of argon, add 0.6 g of m-hydroxybenzaldehyde and 1 g of 2,4-dimethylpyrrole into 150 mL of dichloromethane, stir to dissolve, and then add 0.5 mL of three Fluoroacetic acid was stirred and reacted at room temperature for 10 hours. After the reaction was completed, the solvent was evaporated to dryness and separated with a silica gel column.
[0043] 2) Preparation of formula III: under the protection of argon, 1.2 g of the compound of formula II was dissolved in 100 mL of dichloromethane, and 1.3 g of 2,3-dichloro-5,6 -Dicyano-p-benzoquinone (DDQ) in dichloromethane solution 100 mL, continue to stir the reaction for 4 hours, cool the system in an ice bath, then quickly drop 10 mL triethylamine and 15 mL trifluoride into the reaction system Boron ether solution, continue to stir for 3 h...
Embodiment 2
[0059] (1) Preparation of probe molecules
[0060] Reaction process is with embodiment 1:
[0061] 1) Preparation of formula II: under the protection of argon, add 0.9 g of m-hydroxybenzaldehyde and 1.5 g of 2,4-dimethylpyrrole into 150 mL of dichloromethane, stir to dissolve, and then add 0.5 mL of Tris Fluoroacetic acid was stirred and reacted at room temperature for 12 hours. After the reaction was completed, the solvent was evaporated to dryness and separated with a silica gel column.
[0062] 2) Preparation of formula III: under the protection of argon, 1.8 g of the compound of formula II was dissolved in 100 mL of dichloromethane, and 2.0 g of 2,3-dichloro-5,6 -Dicyano-p-benzoquinone (DDQ) dichloromethane solution 100 mL, continue to stir the reaction for 5 hours, cool the system in an ice bath, then quickly drop 10 mL triethylamine and 23 mL trifluoride into the reaction system Boron ether solution, continue to stir for 3 hours, remove the solvent under reduced pressu...
Embodiment 3
[0078] (1) Preparation of probe molecules
[0079] Reaction process is with embodiment 1.
[0080] 1) Preparation of formula II: under the protection of argon, add 1.2 g of m-hydroxybenzaldehyde and 2 g of 2,4-dimethylpyrrole into 150 mL of dichloromethane, stir to dissolve, and then add 0.5 mL of Tris Fluoroacetic acid was stirred and reacted at room temperature for 15 hours. After the reaction was completed, the solvent was evaporated to dryness and separated with a silica gel column.
[0081] 2) Preparation of formula III: under the protection of argon, 2.4 g of the compound of formula II was dissolved in 100 mL of dichloromethane, and 2.6 g of 2,3-dichloro-5,6 -Dicyano-p-benzoquinone (DDQ) dichloromethane solution 100 mL, continue to stir the reaction for 6 hours, cool the system in an ice bath, then quickly drop 10 mL triethylamine and 30 mL trifluoride into the reaction system Boron ether solution, continue to stir for 4 hours, remove the solvent under reduced pressure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com