Organic boron difluoride complex and preparation method thereof
A technology for boron difluoride and complexes, which is applied in the field of organic boron difluoride complexes and their preparation, can solve the problems of few and few studies on organic boron difluoride complexes, and achieve simple separation and purification of products, chemical Stable properties and large molar absorptivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
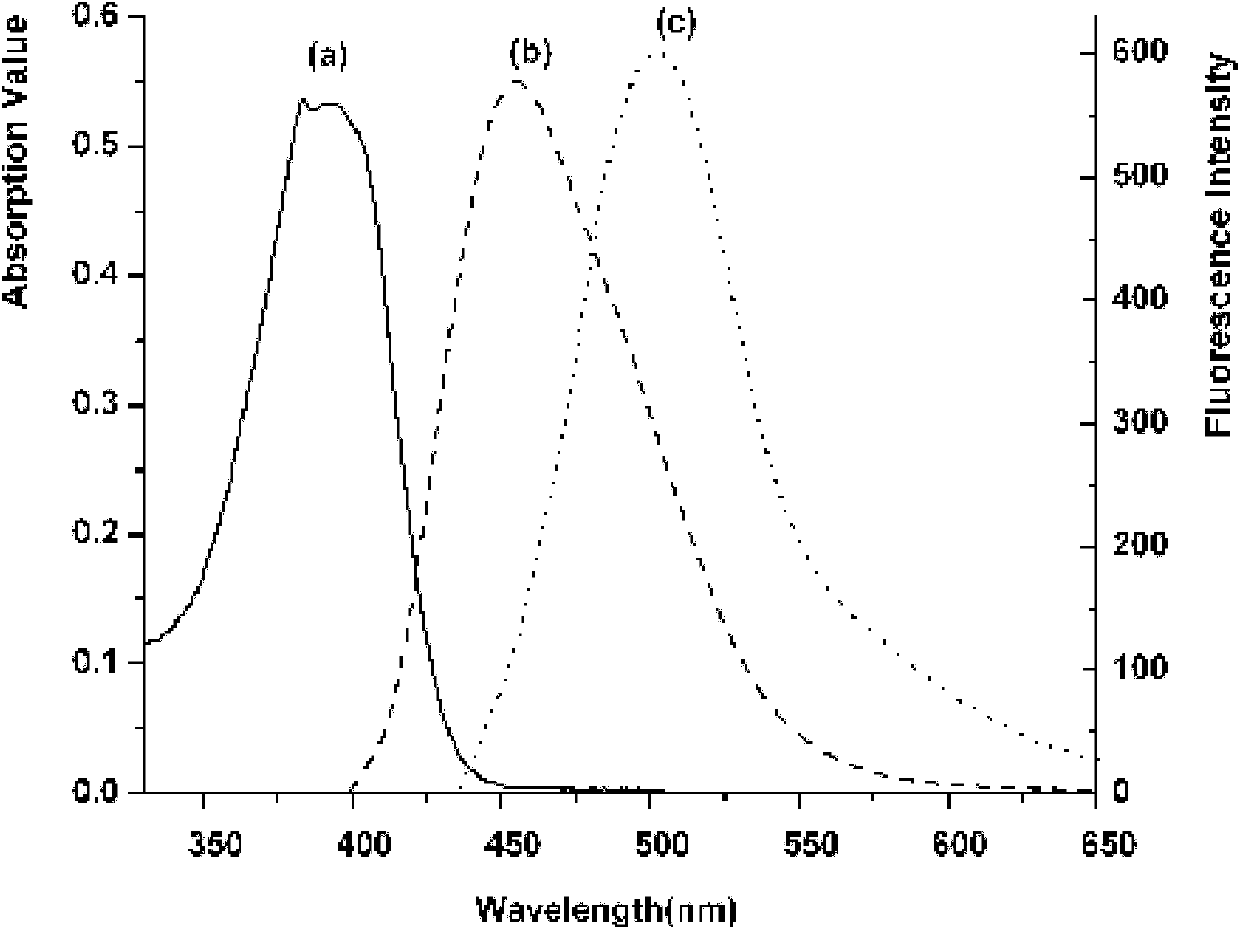
[0034] At room temperature, 2.81g (10mmol) of 2-(6,7-dimethoxy-2-quinolyl)phenol was placed in 15mL of toluene solution, and 4.2mL (30mmol) of triethylamine was added dropwise under stirring, 15 Minutes later, 2.8 mL (30 mmol) of boron trifluoride ether solution was added dropwise, and stirring was continued for 45 min after the addition was complete, gradually solid precipitated out. The solid was collected by filtration, washed repeatedly with anhydrous ether, and naturally dried to obtain complex 1 in the form of light yellow powder with a yield of 81%.
[0035] The infrared spectrum, proton nuclear magnetic resonance spectrum and carbon nuclear magnetic resonance spectrum of the above-mentioned complex 1 are as follows:
[0036] IR(KBr): υ=1603, 1516, 1250, 1083, 849cm -1 .
[0037] 1 H NMR (DMSO-d 6 ): δ=4.02(s, 3H), 4.15(s, 3H), 7.02(t, J=8.0Hz, 1H), 7.07(s, 1H), 7.19(d, J=8.4Hz, 1H), 7.48 (t, J=8.0Hz, 1H), 7.83(d, J=8.0Hz, 1H), 7.94(d, J=8.8Hz, 1H), 8.29(m, 2H).
...
Embodiment 2
[0040]At room temperature, 2.56g (10mmol) of 2-(6-chloro-2-quinolyl)phenol was placed in 15mL of benzene solution, 3.7mL of triethylamine (27mmol) was added dropwise under stirring, and triethylamine was added dropwise after 15 minutes. Boron fluoride ether solution 4.2mL (45mmol), continue to stir for 45min after the addition, gradually solid precipitates out. The solid was collected by filtration, washed repeatedly with anhydrous ether, and naturally dried to obtain complex 2 in the form of yellow powder with a yield of 76%.
[0041] The infrared spectrogram, proton nuclear magnetic resonance spectrum and carbon nuclear magnetic resonance spectrum of above-mentioned complex 2 are as follows:
[0042] IR(KBr): υ=1614, 1517, 1358, 1265, 1165, 1099, 930, 847, 754cm -1 .
[0043] 1 H NMR (DMSO-d 6 ): δ=7.07(t, J=7.6Hz, 1H), 7.20(d, J=8.4Hz, 1H), 7.56(t, J=7.6Hz, 1H), 7.63(d, J=8.4Hz, 1H ), 7.84(d, J=8.8Hz, 1H), 7.90(d, J=8.4Hz, 1H), 8.15(d, J=8.8Hz, 1H), 8.47(d, J=8.8Hz, 1H...
Embodiment 3
[0046] At room temperature, 2.21g (10mmol) of 2-(2-quinolyl)phenol was placed in 10mL of ethyl acetate solution, and 2.9mL (21mmol) of triethylamine was added dropwise with stirring, and trifluorinated trifluoride was added dropwise after 15 minutes. Boron ether solution 2.8mL (30mmol), continue to stir for 45min after the addition, gradually a solid precipitates out. The solid was collected by filtration, washed repeatedly with anhydrous ether, and naturally dried to obtain complex 3 in the form of yellow powder with a yield of 86%.
[0047] The infrared spectrum, proton nuclear magnetic resonance spectrum and carbon nuclear magnetic resonance spectrum of the above-mentioned complex 3 are as follows:
[0048] IR(KBr): υ=1609, 1560, 1523, 1481, 1265, 1085, 922, 760cm -1 .
[0049] 1 H NMR (DMSO-d 6 ): δ=7.06(t, J=7.6Hz, 1H), 7.21(d, J=8.4Hz, 1H), 7.54(t, J=8.0Hz, 1H), 7.69(t, J=7.6Hz, 1H ), 7.91 (m, 3H), 8.18 (d, J=8.8Hz, 1H), 8.52 (d, J=8.8Hz, 1H), 8.96 (d, J=8.8Hz, 1H)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com