Benzothiazole derivative with optical spectrum signal amplification performance and preparation and application thereof
A benzothiazole and signal amplification technology, which is applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve problems such as small absorption coefficient, and achieve the effect of large molar absorption coefficient and high fluorescence quantum yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
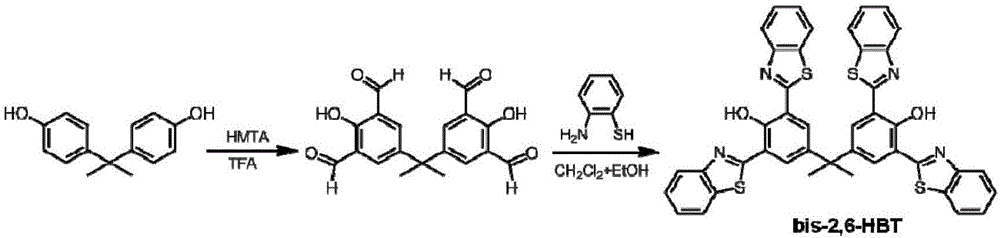
[0024] Synthesis of benzothiazole derivatives. The synthetic route of bis-2,6-HBT is as follows figure 1 shown.
[0025] (1) Under nitrogen protection, after bisphenol A (4.5g) and hexamethylenetetramine (18g) were refluxed for 72h in 30ml trifluoroacetic acid and under the condition of 105-115°C, 90ml of hydrochloric acid solution was added ( 2M), continue to react for 1h, and cool to room temperature. The solid crude product was obtained by filtration and further purified by column chromatography with dichloromethane as the eluent. The solvent was removed by rotary evaporation to obtain 4.0 g of solid product with a yield of 59%.
[0026] 1 H NMR (400MHz, DMSO), δ (ppm): 11.52 (s, 2H), 10.22 (s, 4H), 7.94 (s, 4H), 1.72 (s, 6H); 13 C NMR (100MHz, DMSO), δ (ppm): 30.37, 41.99, 123.67, 135.50, 141.51, 159.34, 160.99, 192.90.
[0027] (2) Under nitrogen protection, the product obtained in step (1) bisphenol A tetraaldehyde (0.3g) and 2-aminothiophenol (0.6g) were dissolve...
Embodiment 2
[0030] Measurement and comparison of spectral signal parameters.
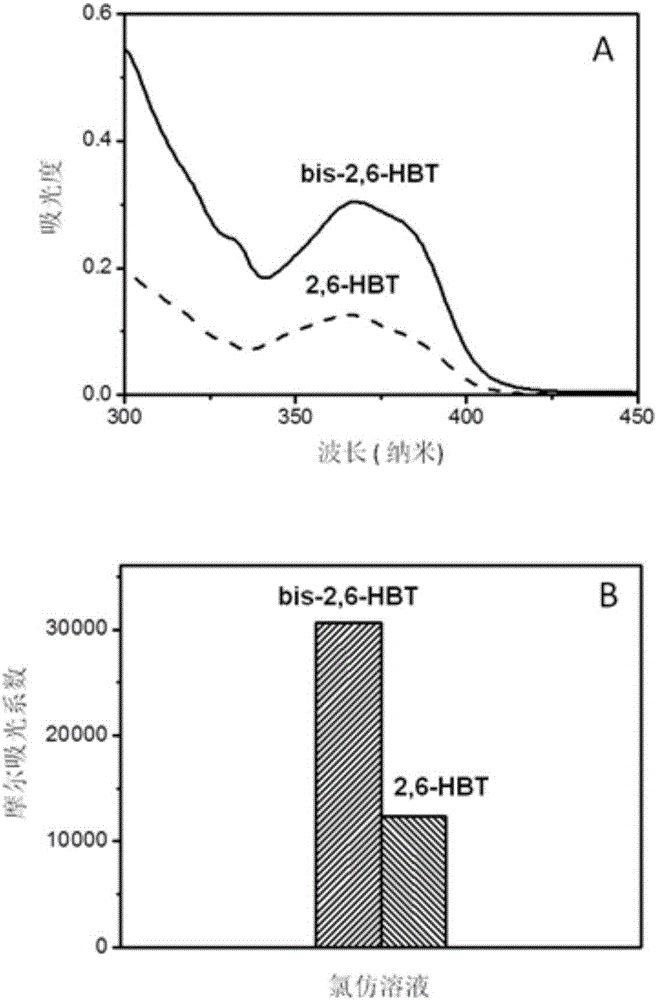
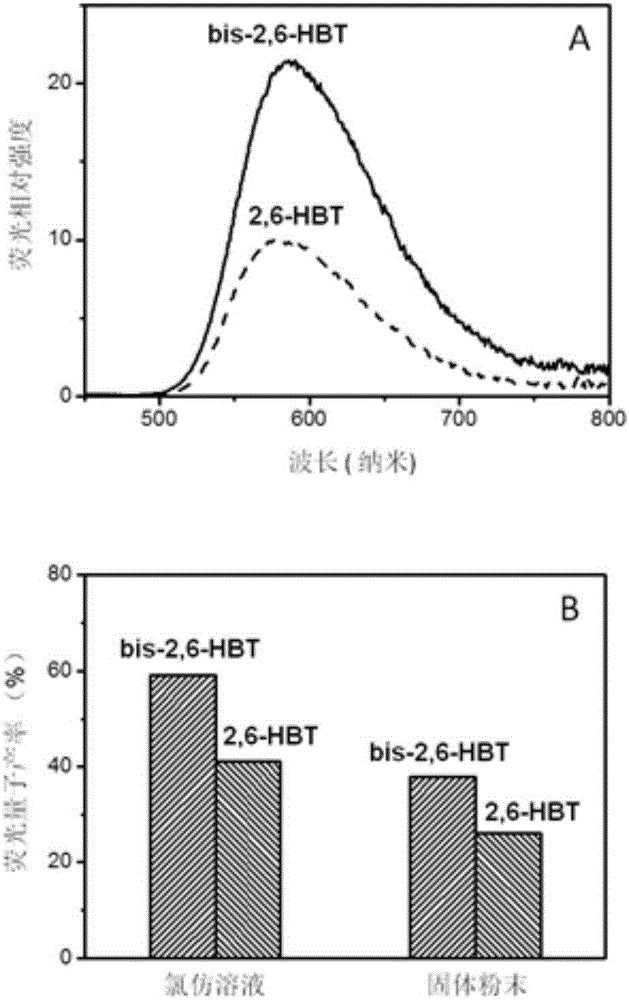
[0031] The benzothiazole derivative bis-2,6-HTB obtained in Example 1 and the comparative compound 2,6-HBT were respectively dissolved in chloroform to prepare a 10 μM / l solution. Pipette the above-mentioned chloroform solution of 3ml and be placed in quartz cuvette, test absorption spectrum on PerkinElmerLambda 35 spectrometer, calculate molar absorptivity according to absorbance ( figure 2 ). Pipette 3ml of the above-mentioned chloroform solution and place it in a fluorescence cuvette, and test the fluorescence spectrum on an Edinburgh FS5 spectrometer ( image 3 A). The fluorescence quantum yields of chloroform solution and solid powder samples are obtained by direct measurement of the absolute value using the integrating sphere accessory configured by Edinburgh FS5, and the results are compared as image 3 Shown in B. It can be seen from the test results that the spectrum of the benzothiazole derivativ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar absorption coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com