Phenylvinyl-BODIPY derivative and preparation method thereof
A technology of phenylvinyl and derivatives, which is applied in the field of organic materials, can solve the problems of insufficient research on the synthesis methodology of phenylvinyl BODIPY, and achieve favorable ion complexation and biological dyeing, convenient synthesis, and high molar light absorption The effect of the coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0045] Preparation of Compound 1-10
[0046] 1. The general synthetic method of compound 1-10 (unless otherwise specified)
[0047] Add 0.1 mmol (or 0.2 mmol) of the corresponding BODIPY and 0.35 equivalent of aromatic aldehyde to a microwave tube, dissolve the substrate with 2ML (or 4ML) dry DMF, then catalyst: 50 μL (or 100 μL) piperidine and 50 μL (or 100 μL) acetic acid was added to the reaction mixture. Using Biotage microwave reactor, react at 130°C for 10-45Min. The solvent was then rotovapped using an oil pump as a vacuum source. Finally, the target compound is purified by column chromatography or thin layer chromatography.
[0048]
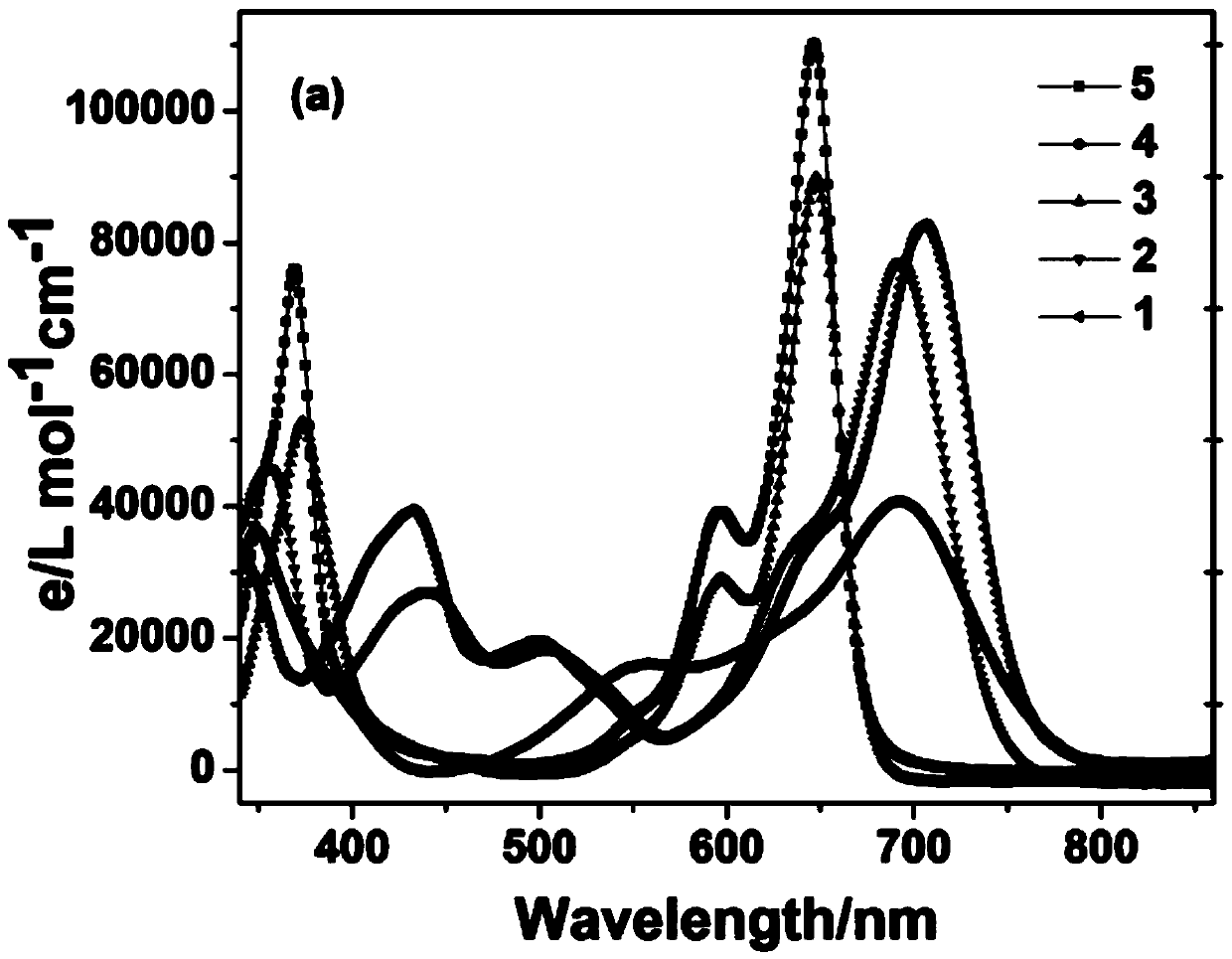
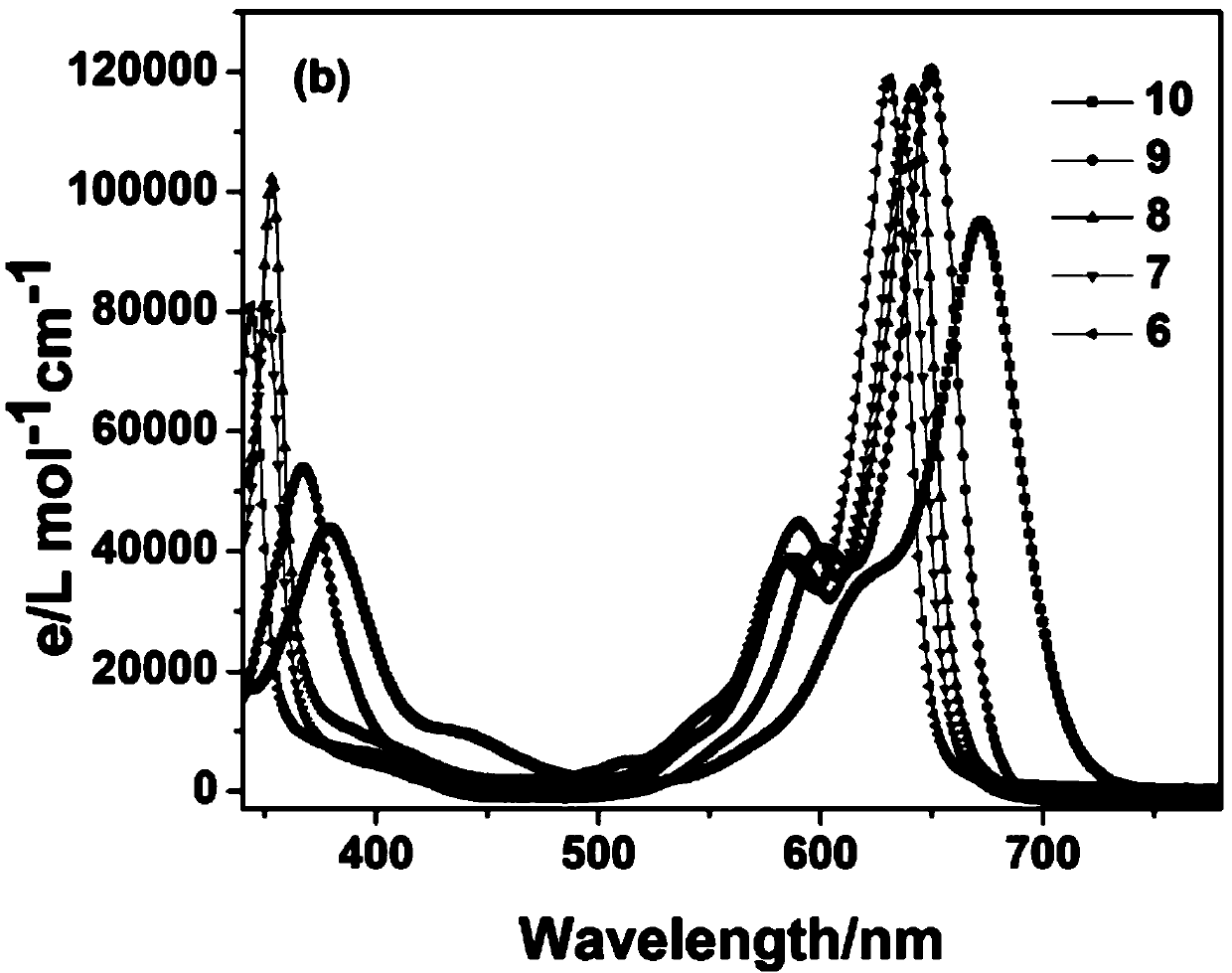
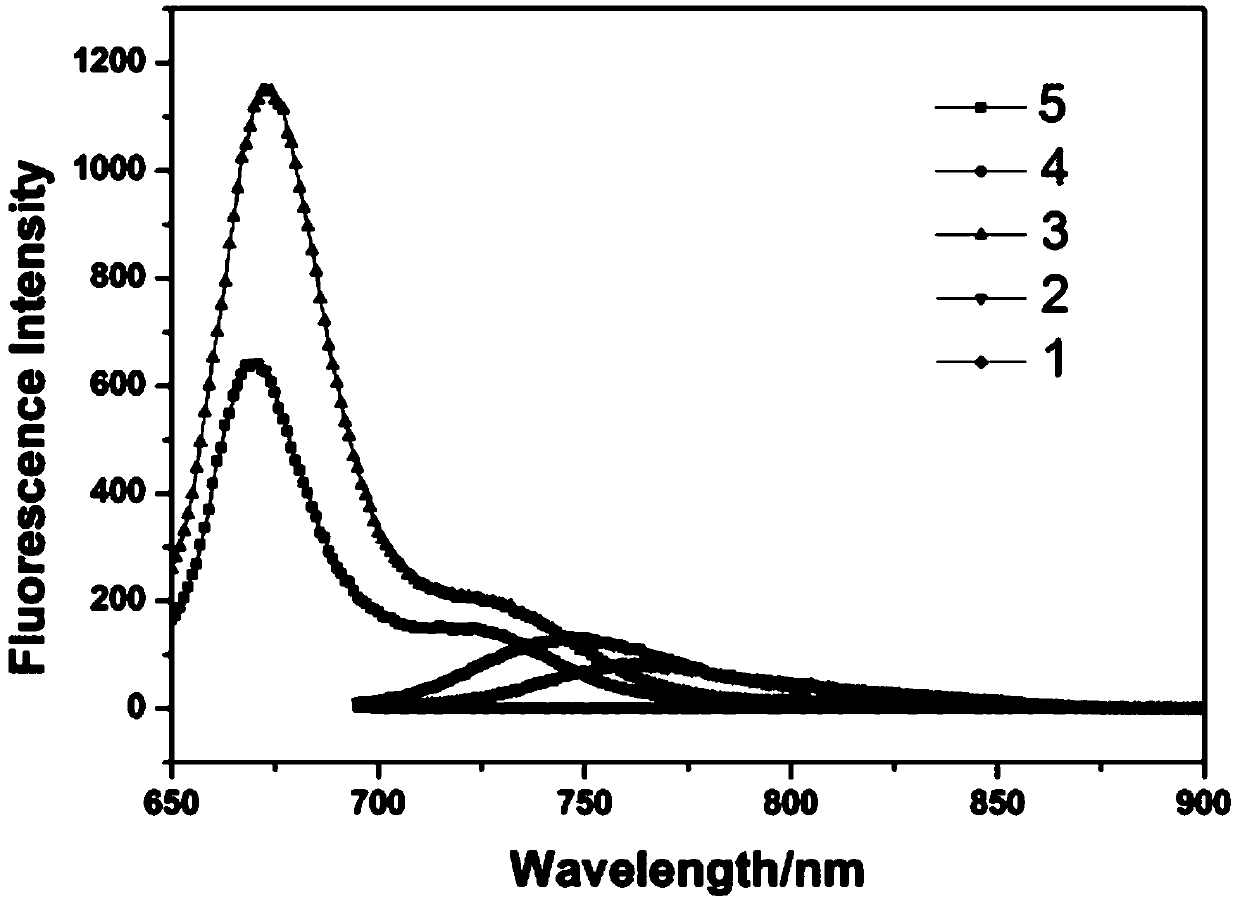
[0049] image 3 Structure and Yield of Compound 1-10
[0050]Two, the specific synthetic steps, reaction rate and yield of compound 1-10
[0051] Compound 1: BODIPY A 0.1 mmol was reacted with 4-N,N-dimethylaminobenzaldehyde for 45 Min to obtain 46.5 mg of Compound 1, yield: 65.3%. 1 H NMR (CDCl 3 ,400MHz)δ7.84(2H,d,J=8.3Hz,Ar-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com