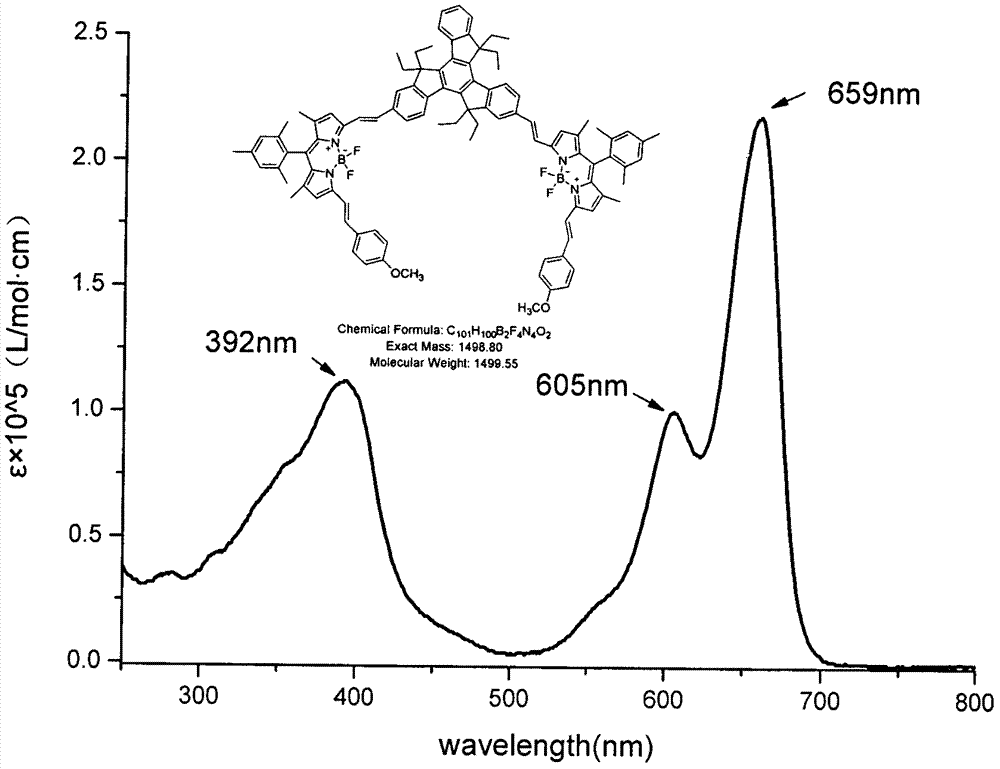
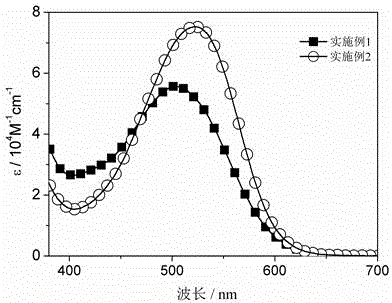
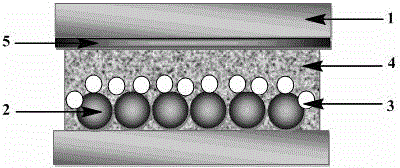
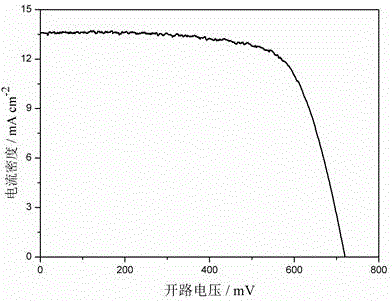
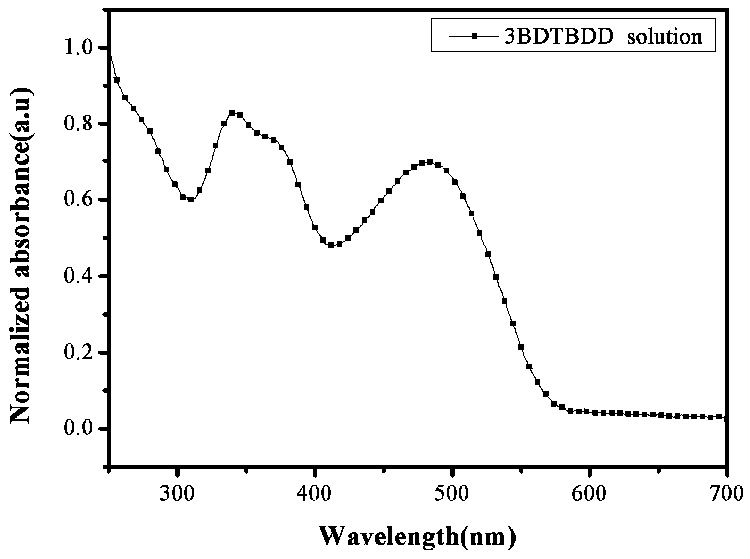
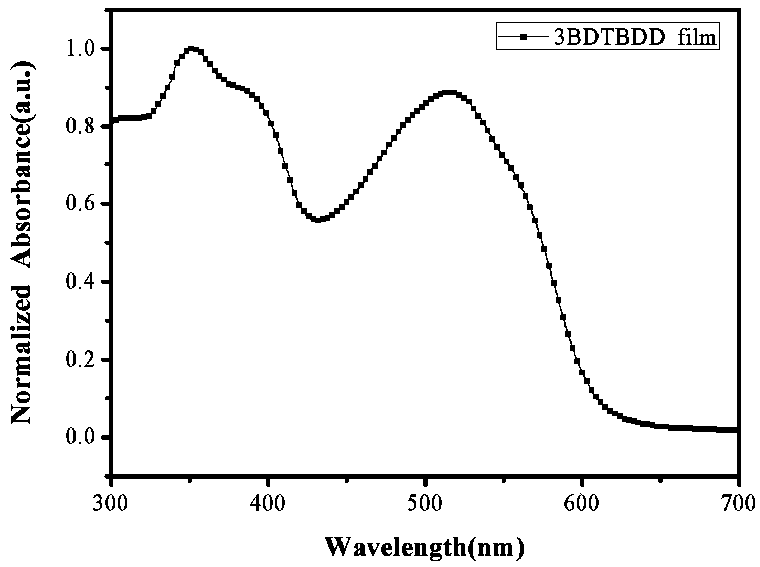
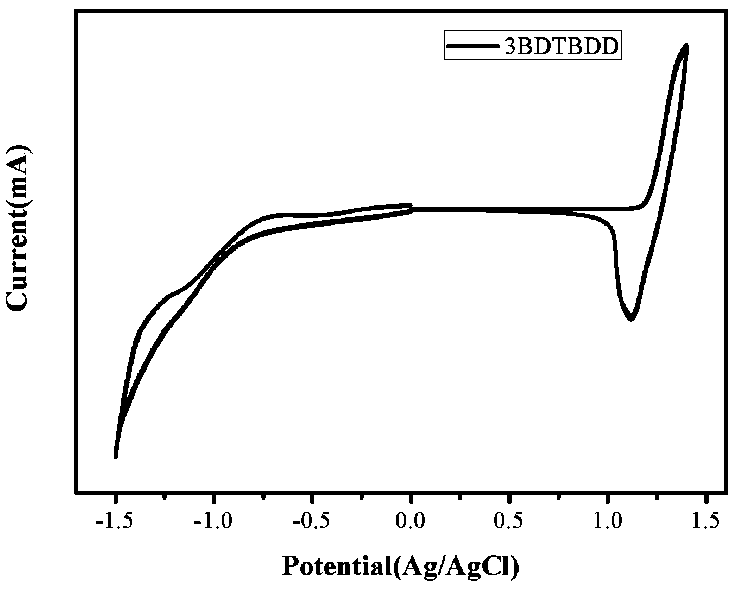
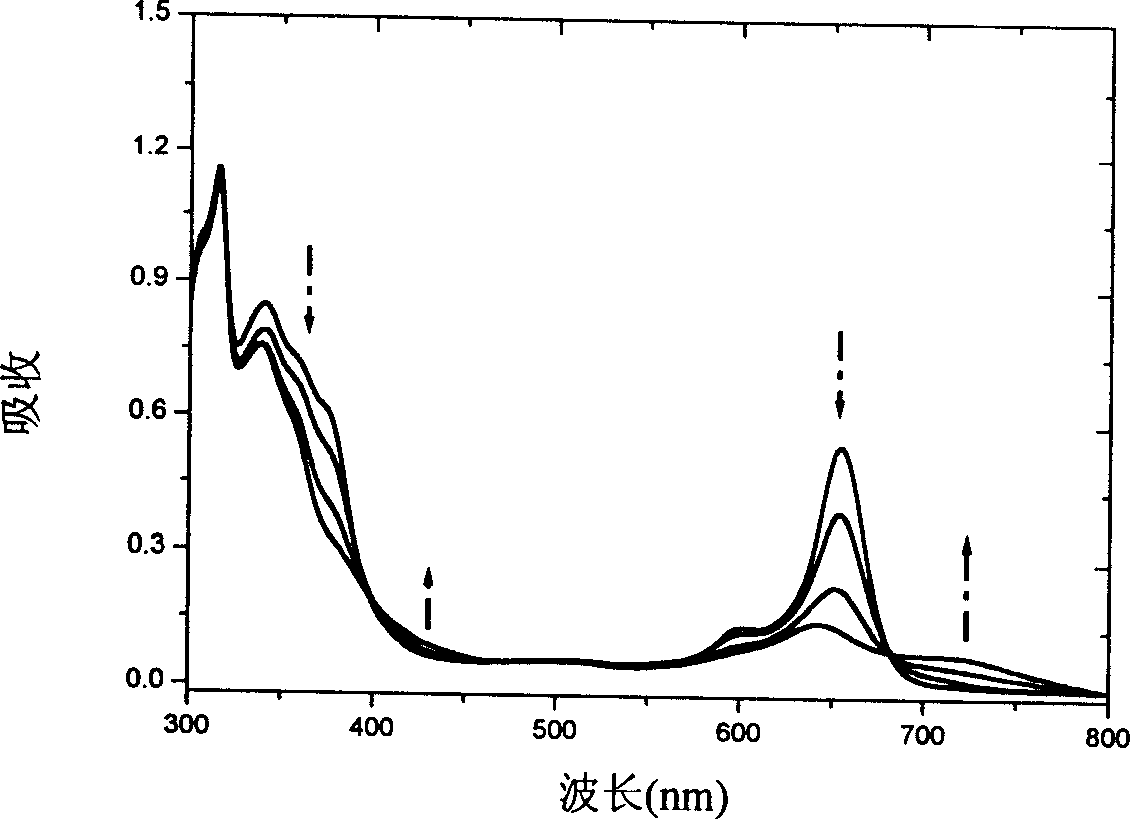
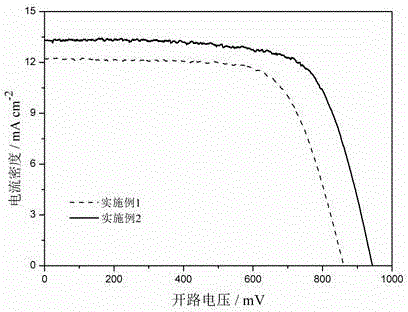
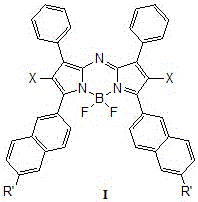
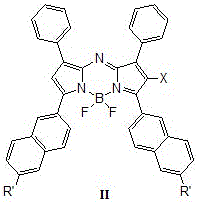
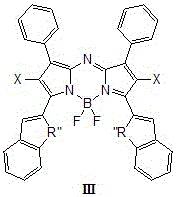
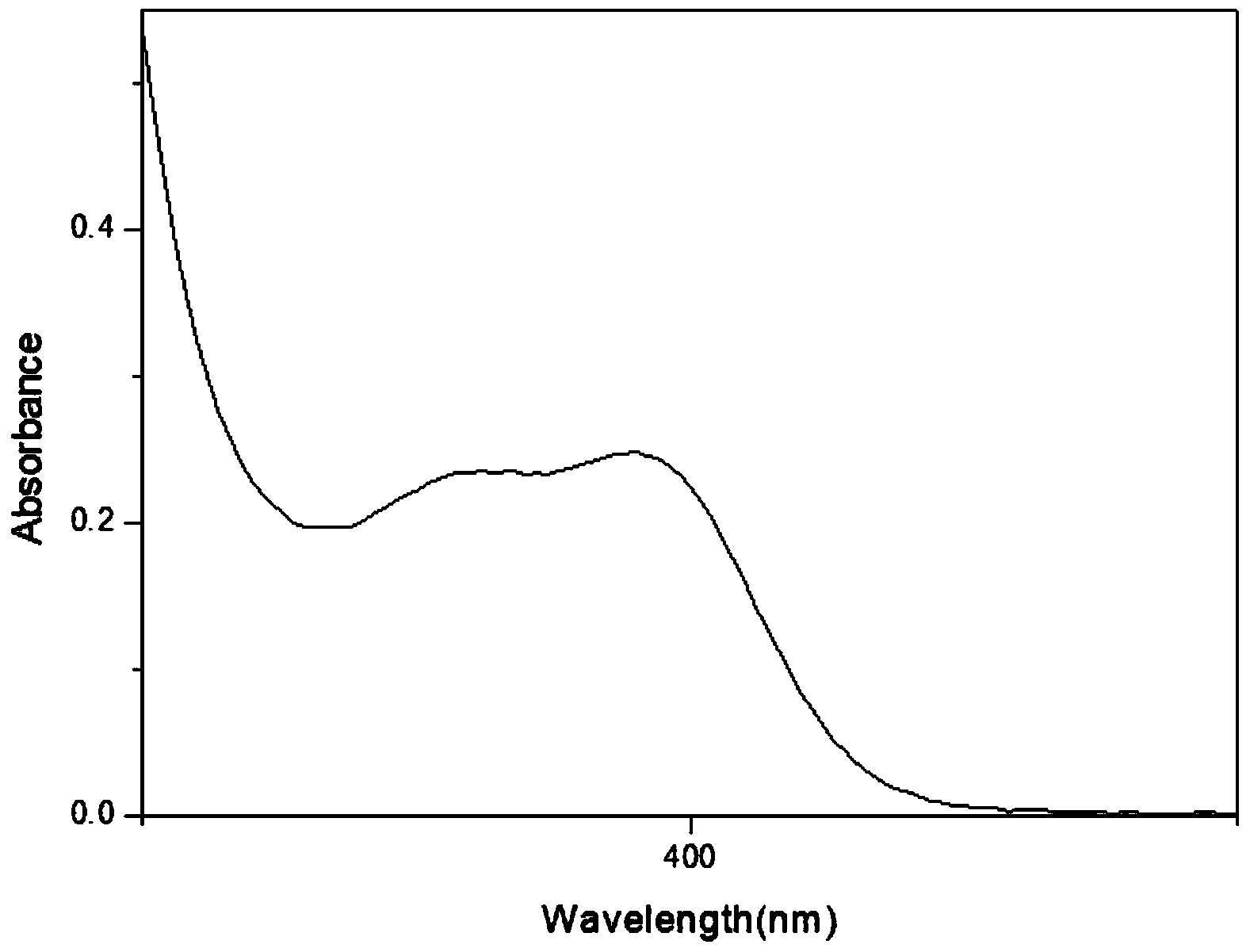
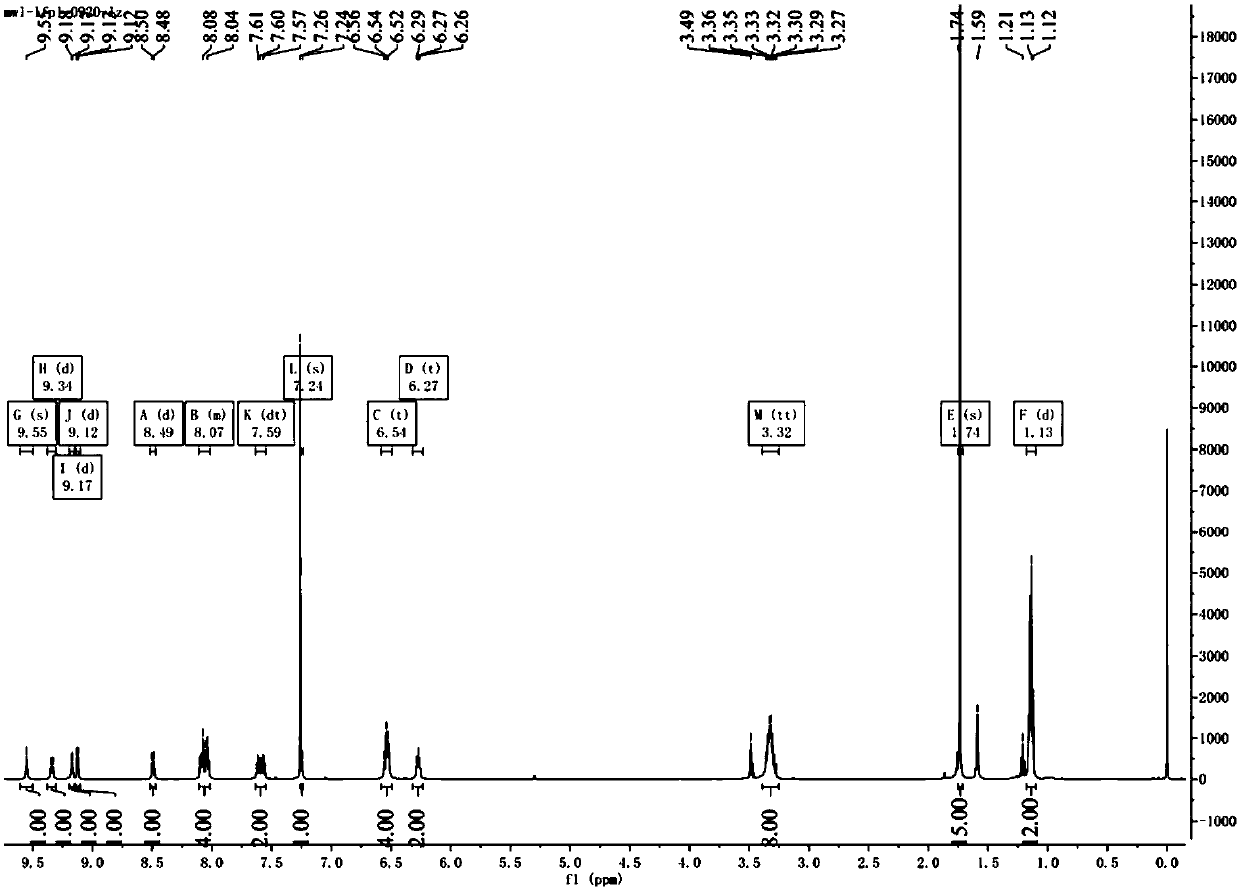
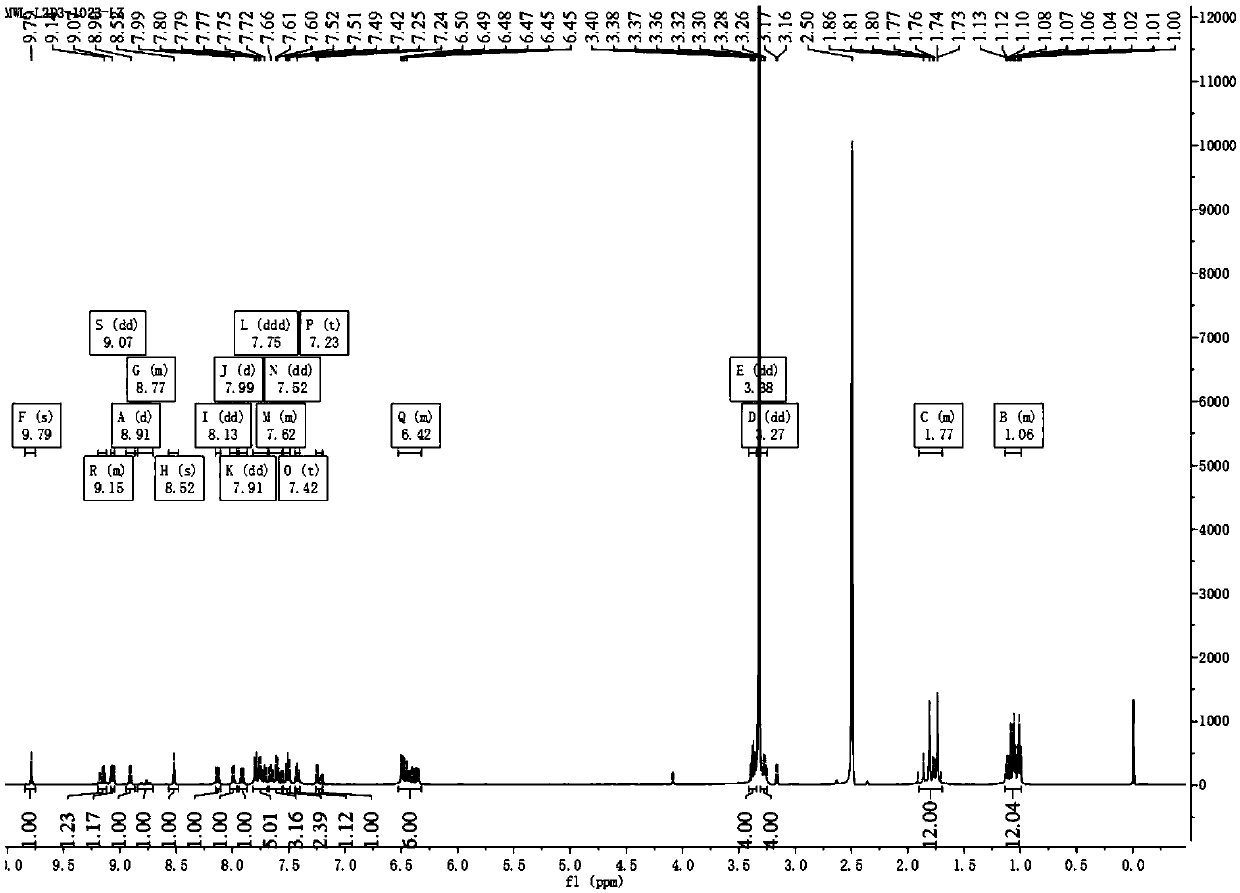
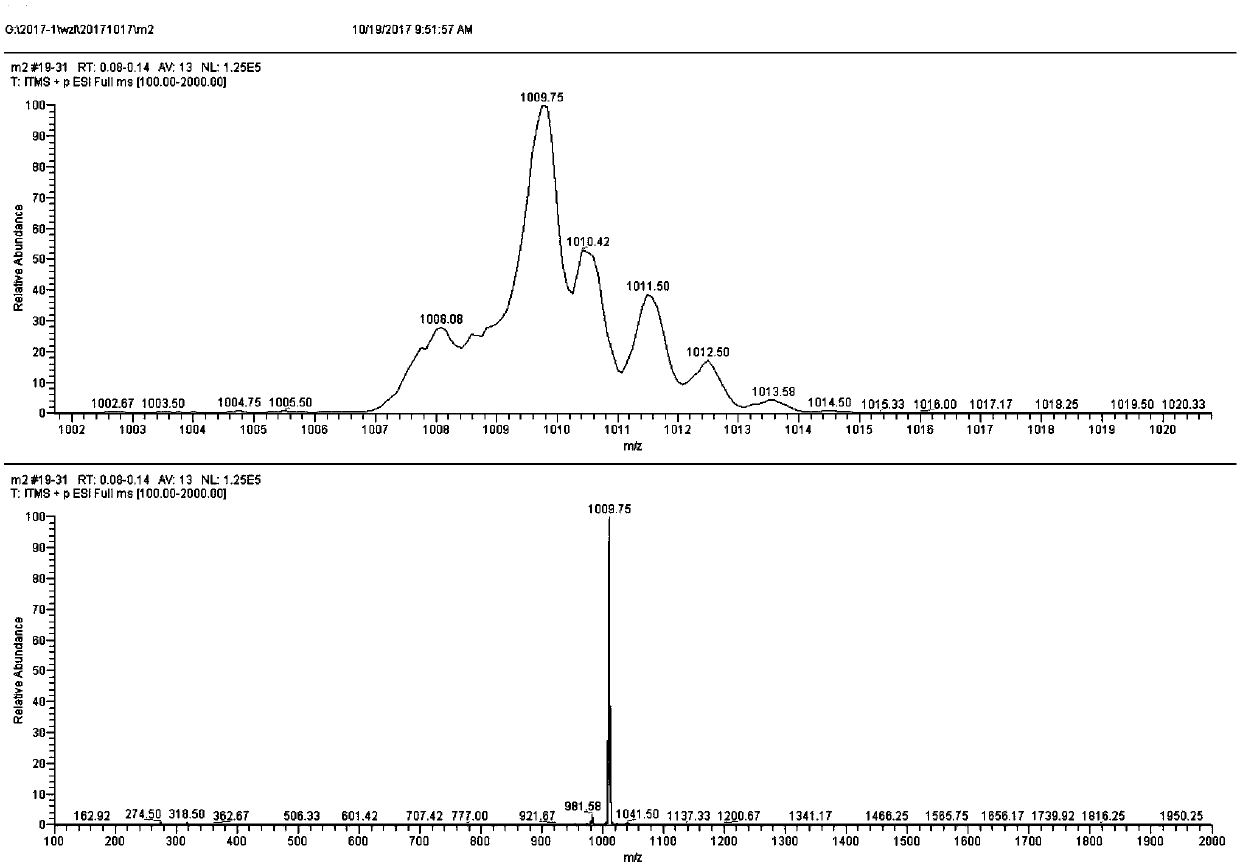
Patents
Literature
96results about How to "High molar absorptivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Filter and Cyanine Compound
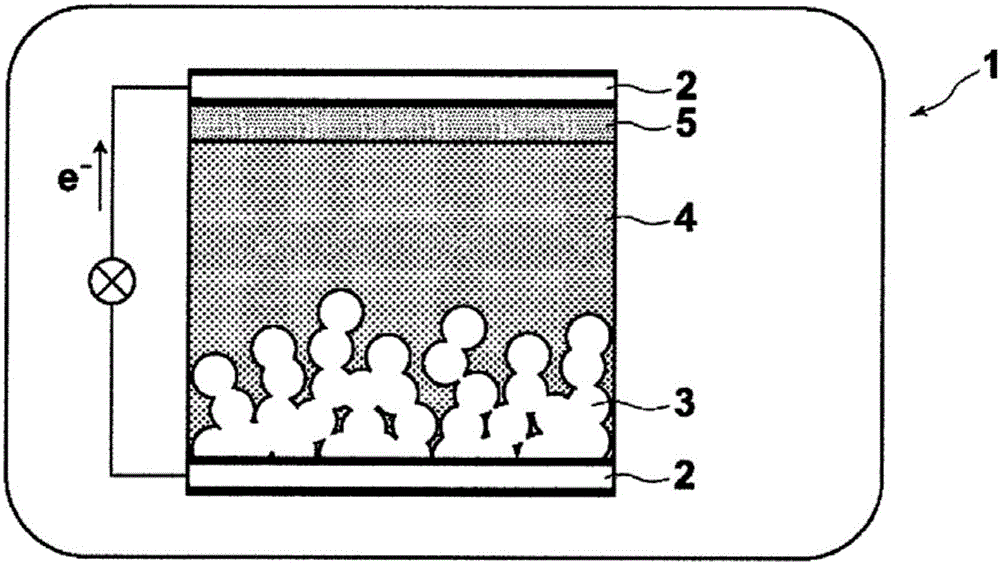
InactiveUS20080048155A1High molar absorptivityImprove heat resistanceMethine/polymethine dyesOrganic chemistryDisplay deviceMoisture resistance
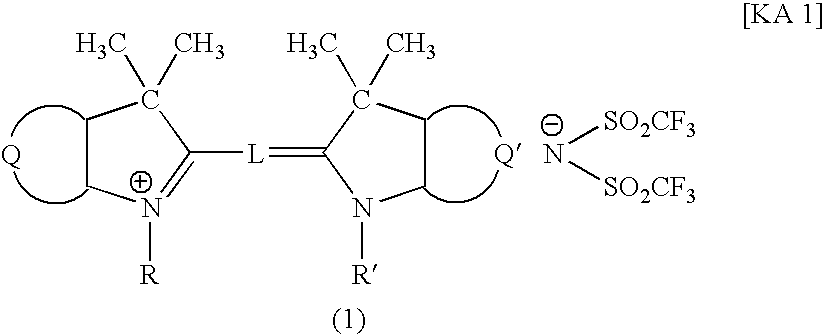
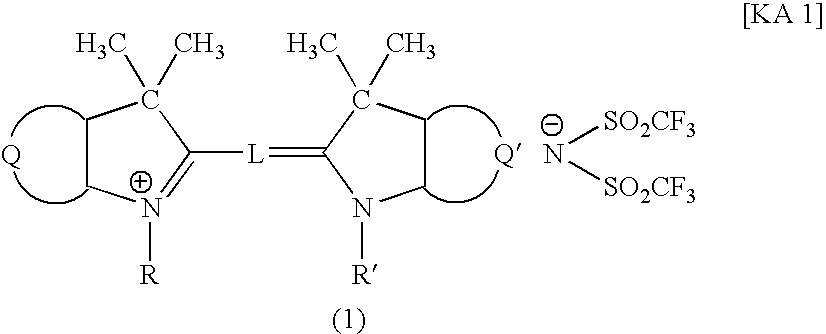
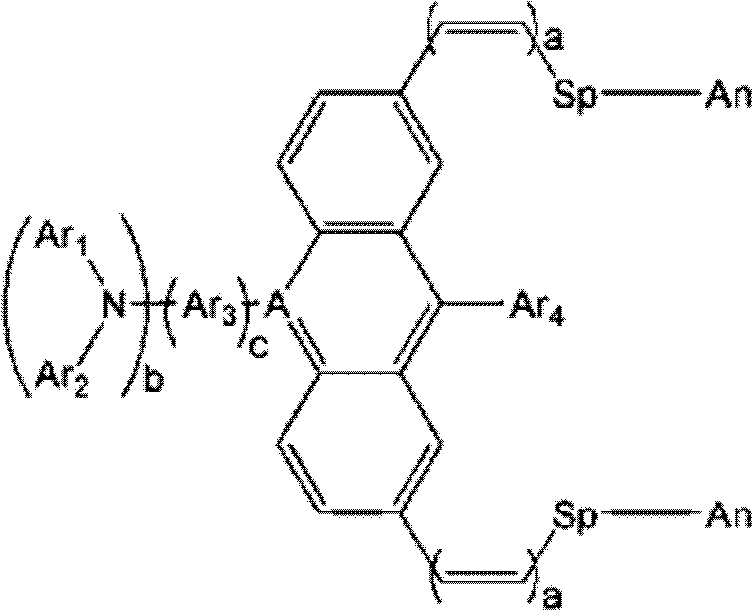
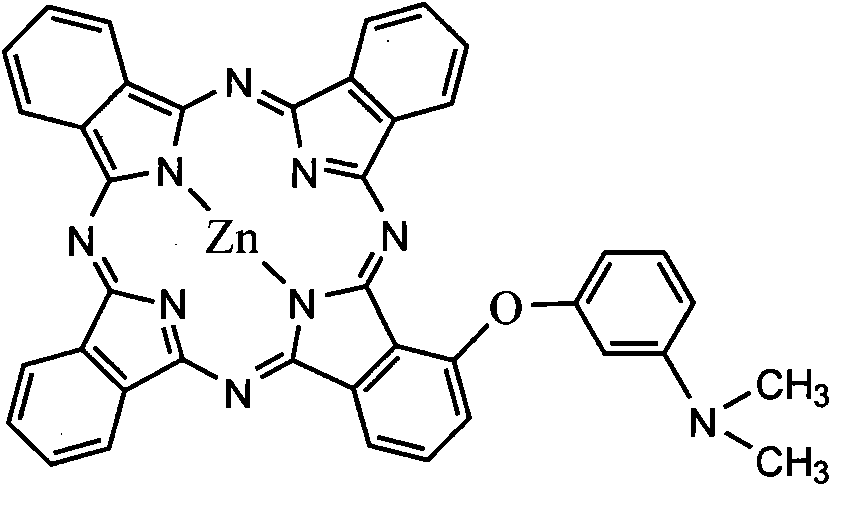
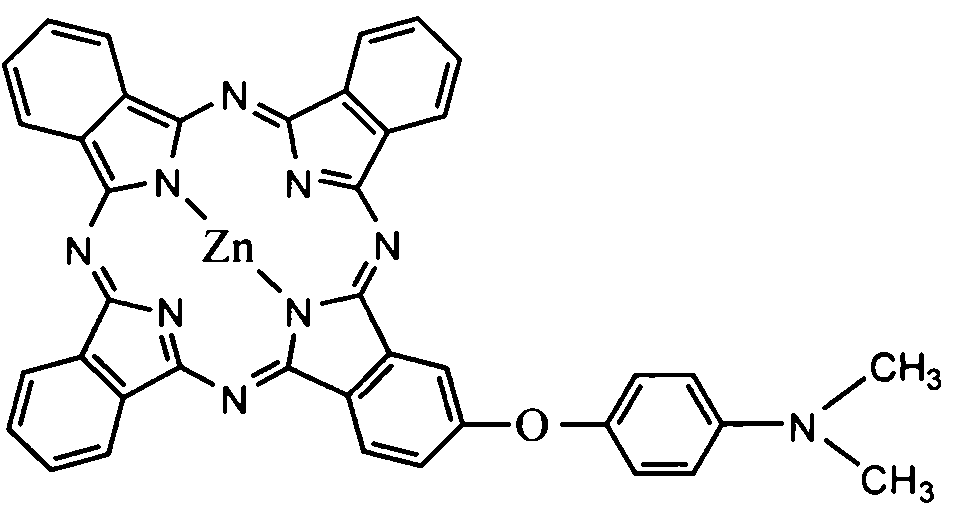
An optical filter, which is capable of cutting-off unnecessary near infrared rays, neon light or the like, and preventing reflection of fluorescent light or the like, in plasma display panels and the like, at the same time, superior in transmittance for visible light, and also superior in heat resistance, moisture resistance, and the like of the coloring matter contained, had been desired. The present invention was made to solve such problems. The present invention relates to an optical filter comprising a salt of a cation of a cyanine coloring matter with a di(halogenoalkylsulfonyl)imide anion, preferably a cyanine compound represented by the following formula: (wherein, in the above formula (1), each of Q and Q′ independently represents a benzene ring or a naphthalene ring which may have a substituent; each of R and R′ independently represents an alkyl group or an alkoxyalkyl group; and L represents a linking group to form a carbocyanine), and useful as a filter for cutting-off near infrared rays, improving image characteristics for displays or the like.
Owner:NIPPON KAYAKU CO LTD
Colored curable composition, resist liquid, ink for inkjet printing, color filter, method of producing color filter, solid-state image sensor, liquid crystal display, organic el display, image display device and colorant compound
ActiveUS20120264039A1Excellent color purity and fastness and pattern formation propertySuppressed color transferRadiation applicationsOptical filtersLiquid-crystal displayDisplay device
The invention provides a colored curable composition including a dipyrromethene compound having a structure in which a polymerizable group and a carboxyl group are introduced in the same molecule, a resist liquid, an ink for inkjet printing, a color filter, a method of producing a color filter, a solid-state image sensor, a liquid crystal display, an organic EL display, an image display device and a colorant compound.
Owner:FUJIFILM CORP
Near-infrared truxene-based conjugate dual-BODIPY fluorescent dye and preparation method thereof
ActiveCN106928262ANarrow absorbencyNarrow emission peakAzo dyesGroup 3/13 element organic compoundsSolubilityFluorescence
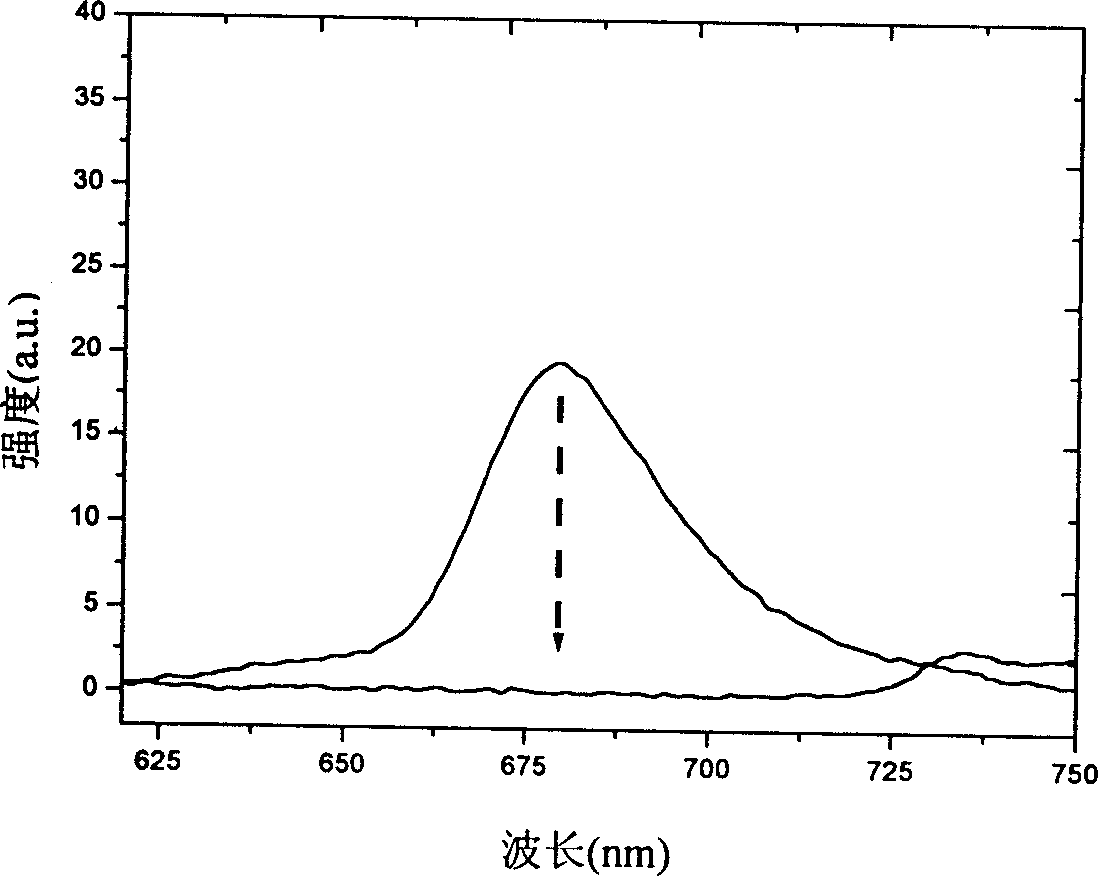
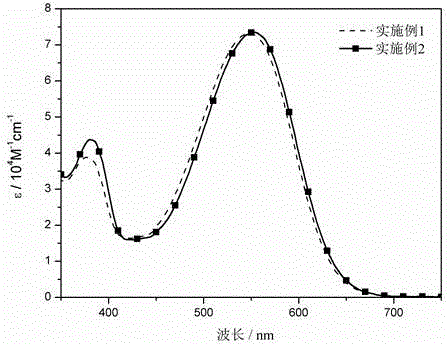
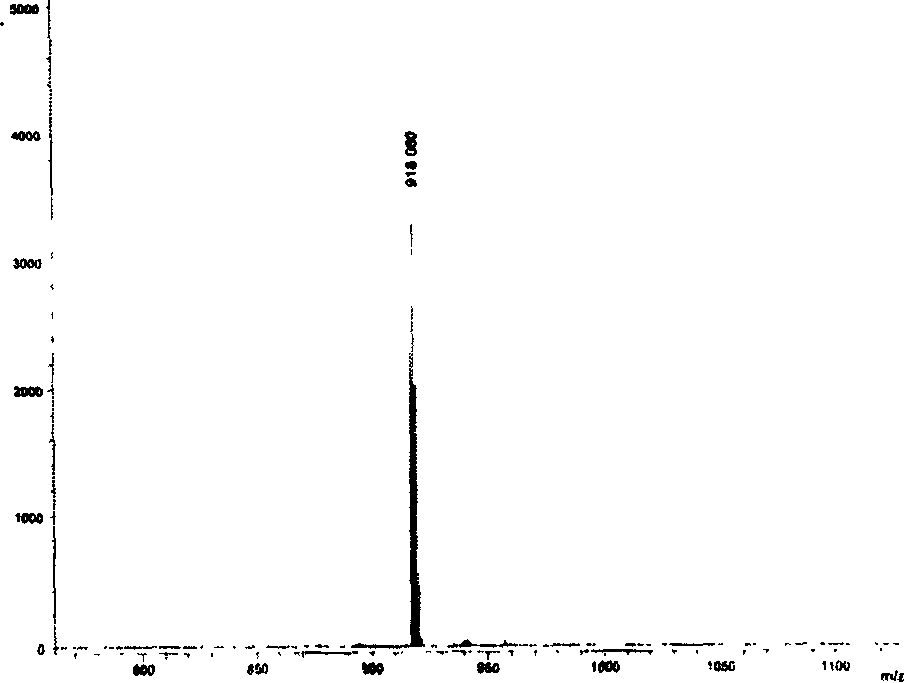
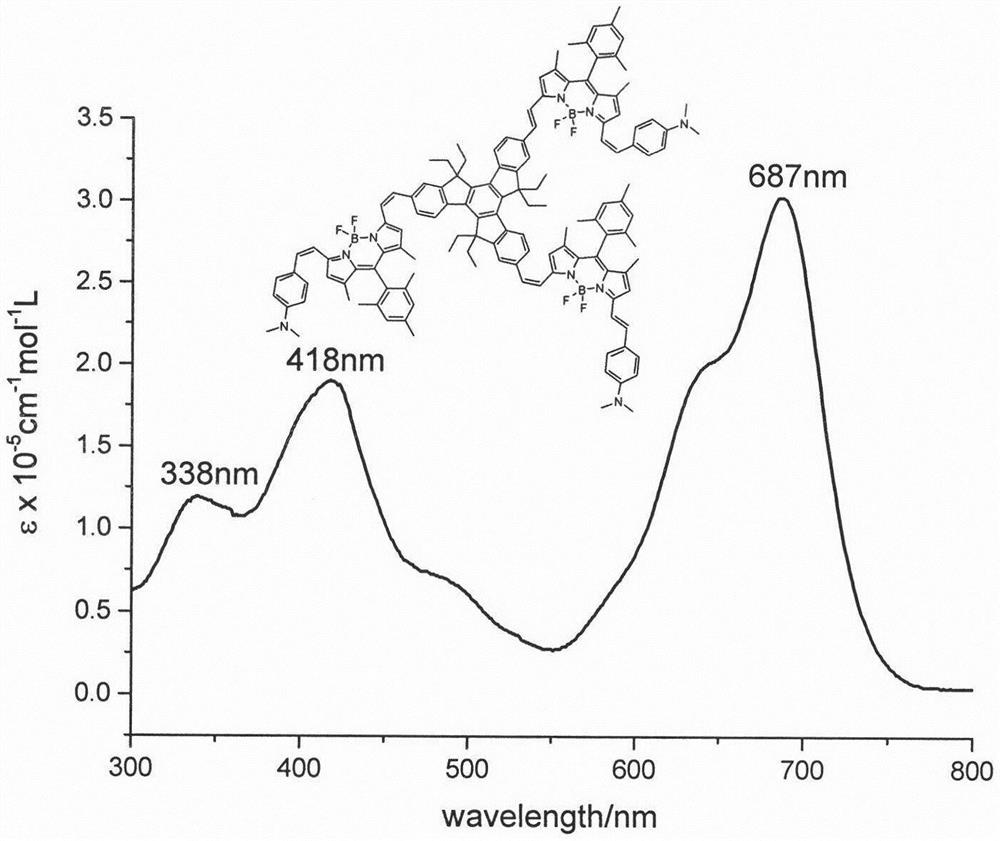
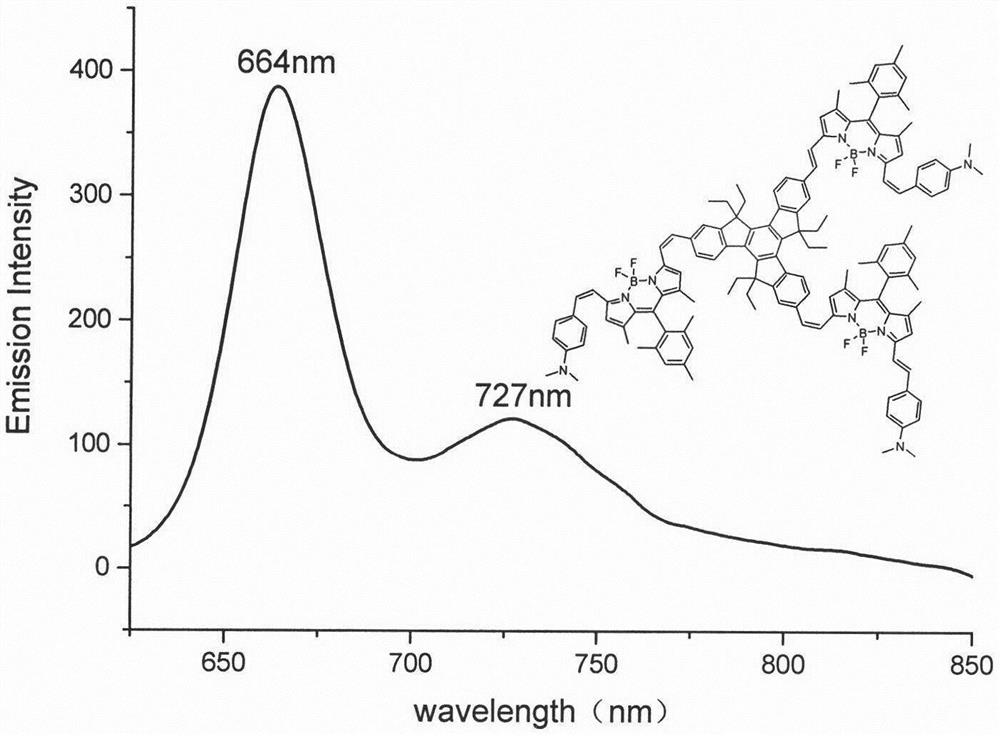
The invention relates to a near-infrared truxene-based conjugate dual-BODIPY fluorescent dye and a preparation method thereof. The fluorescent dye is synthesized by virtue of Knoevenagel condensation reaction of a BODIPY derivative and dialdehyde-containing truxene under the catalysis of p-toluenesulfonic acid and piperidine. The preparation method has the beneficial effects of simple reaction steps, mild reaction conditions and relatively good selectivity. The fluorescent dye has excellent physical properties of relatively high molar extinction coefficients, good solubility and light stability and the like, the highest electronic absorption spectrum red shift reaches above 650nm, the fluorescence emission wavelength reaches 680nm, and the fluorescent dye has very high application prospects. The fluorescent dye has good potential application prospects in the fields of cell imaging, bio-labeling or photoelectric materials and the like.
Owner:南京颐维环保科技有限公司
Triphenylamine-thiophene organic dyestuff as well as preparation method and application thereof
InactiveCN103554957AInhibitory complexIncrease the open circuit voltageLight-sensitive devicesOrganic chemistryOrganic dyeThiophene derivatives
The invention provides a triphenylamine-thiophene organic dyestuff. The structural general formula of the triphenylamine-thiophene organic dyestuff is as shown in a formula (I), wherein in the formula (I), R1 is C1-C6 alkoxy, the structural formula of Ar is as shown in a formula (II) or a formula (III), and R2 in the formulas (II) and (III) is C1-C6 alkyls. A preparation method of the triphenylamine-thiophene organic dyestuff comprises the following steps: sequentially carrying out Suzuki coupled reaction and Knoevenagel condensation reaction by using aryl bromal, tetrakis(triphenylphosphine)palladium(0), potassium carbonate and alkoxy substituted triphenylamine boron ester as raw materials, thus obtaining a target product. The triphenylamine-thiophene organic dyestuff can serve as a photosensitizer of a dye-sensitized solar cell. The triphenylamine-thiophene organic dyestuff has the advantages that the distortion spatial structure of alkyl triphenylamine is capable of effectively suppressing the electron recombination and increasing the open-circuit voltage; a thiophene derivative serving as a dyestuff molecule conjugated bridge is capable of ensuring the high molar absorption coefficient and has relatively strong power supply performance, so that the ideal photoelectric conversion efficiency is ensured.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Novel Organic Dye And Preparation Method Thereof
InactiveCN102803394AImprove efficiencyHigh molar absorptivityMonoazo dyesMethine/polymethine dyesOrganic dyePhotoelectric conversion
The present invention relates to a novel organic dye for a dye-sensitized photoelectric conversion device, and a preparation method thereof. The organic dye according to the present invention is used as a dye-sensitized photoelectric conversion device in a dye-sensitized solar cell (DSSC) and provides improved molar absorptivity, Jsc (short circuit photocurrent density) and photoelectric conversion efficiency when compared with known dyes, thereby being capable of remarkably improving the efficiency of the solar cell.
Owner:DONGJIN SEMICHEM CO LTD
Organic boron difluoride complex
InactiveCN102993224AChemically stableImprove thermal stabilityGroup 3/13 element organic compoundsLuminescent compositionsLaser dyePhotosensitizer
The invention discloses an organic boron difluoride complex. The complex is shown in the structural formula I in the specification, wherein R1 is H or OCH3; and R2 is C6H5, 4-HOC6H4, 4-(CH3)2NC6H4 or 2-C4H4S. The complex can be used for preparing luminescent materials, fluorescent probes, fluorescent tracers, information storage media, laser dyes and photodynamic cancer treatment photosensitizers. The invention also discloses a preparation method of the complex. The complex is prepared through reaction of a corresponding ligand and boron trifluoride diethyl etherate. The complex has the advantages of mild reaction conditions, short reaction time, high product yield, rapidness, simpleness and convenience in separation and purification and high product purity.
Owner:ZHEJIANG SCI-TECH UNIV
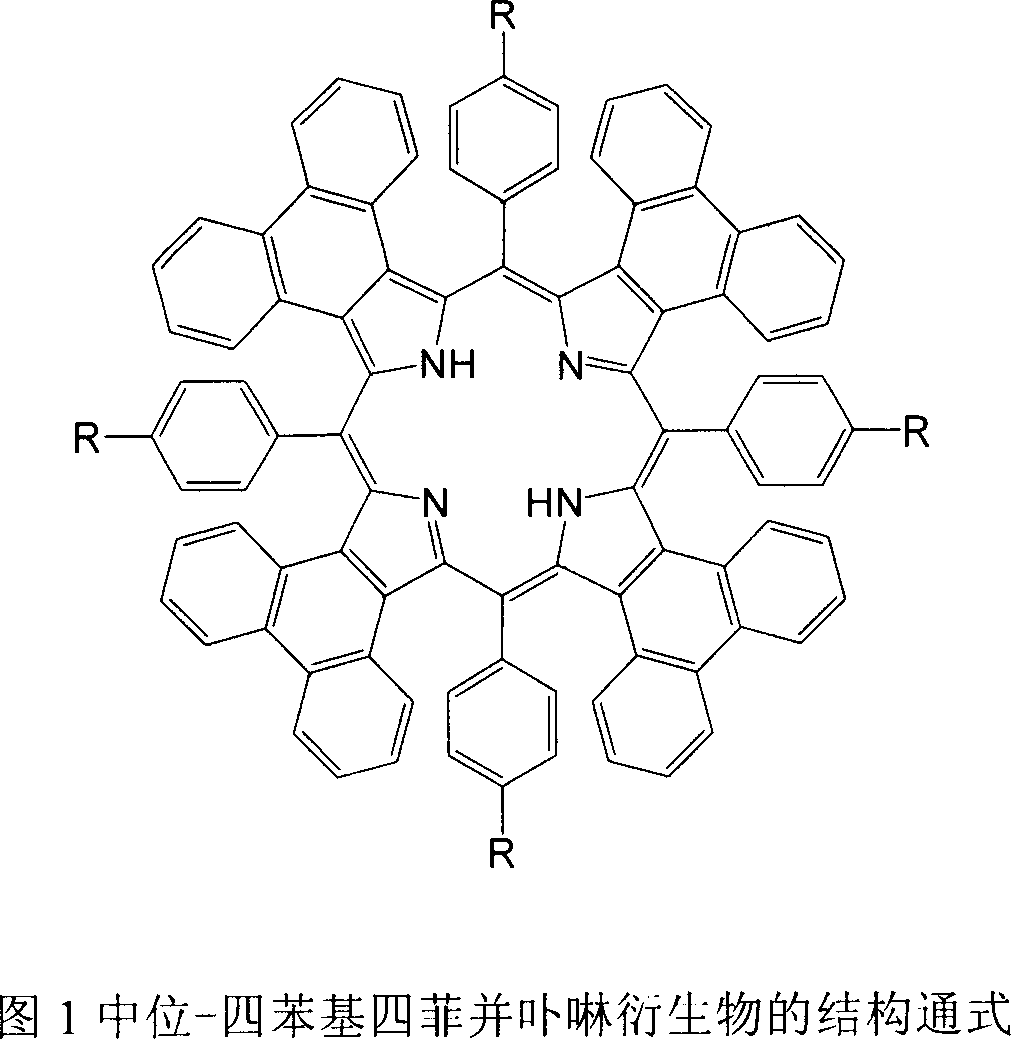
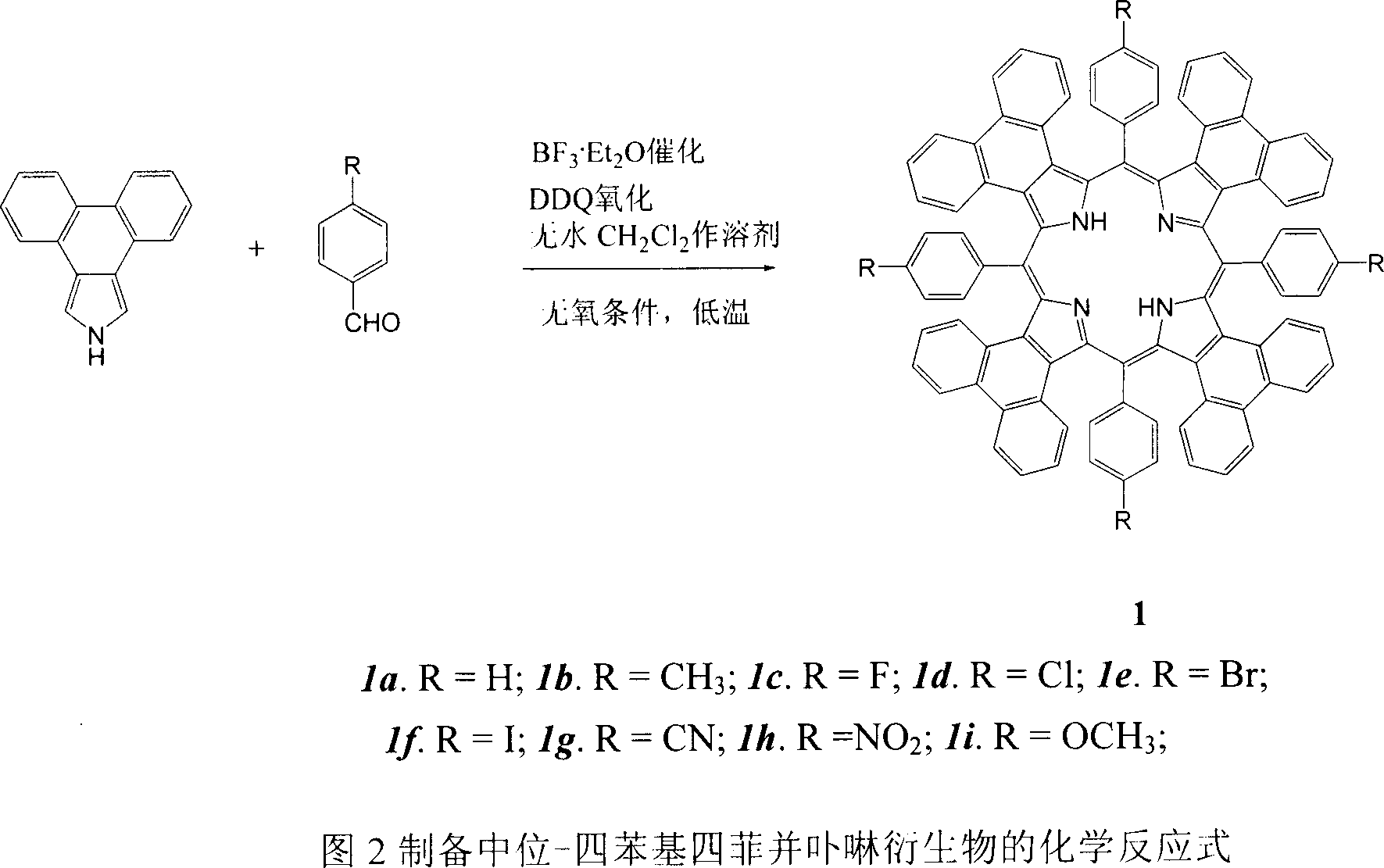
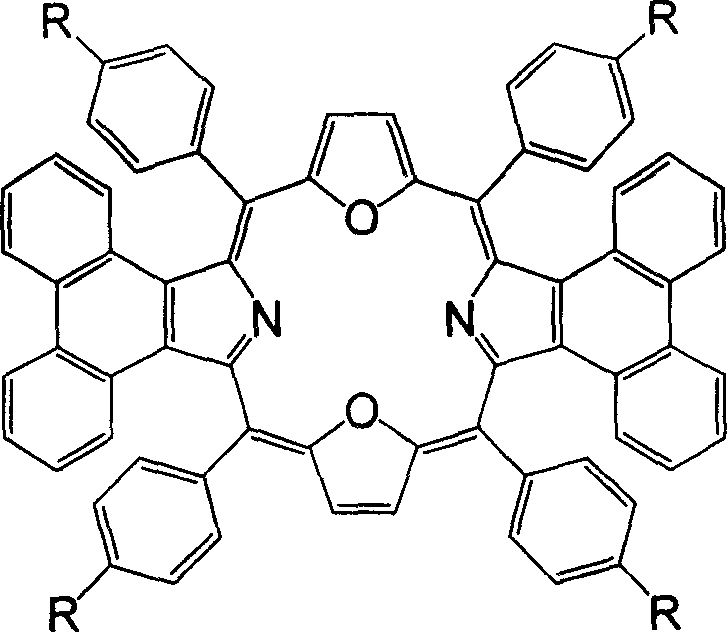
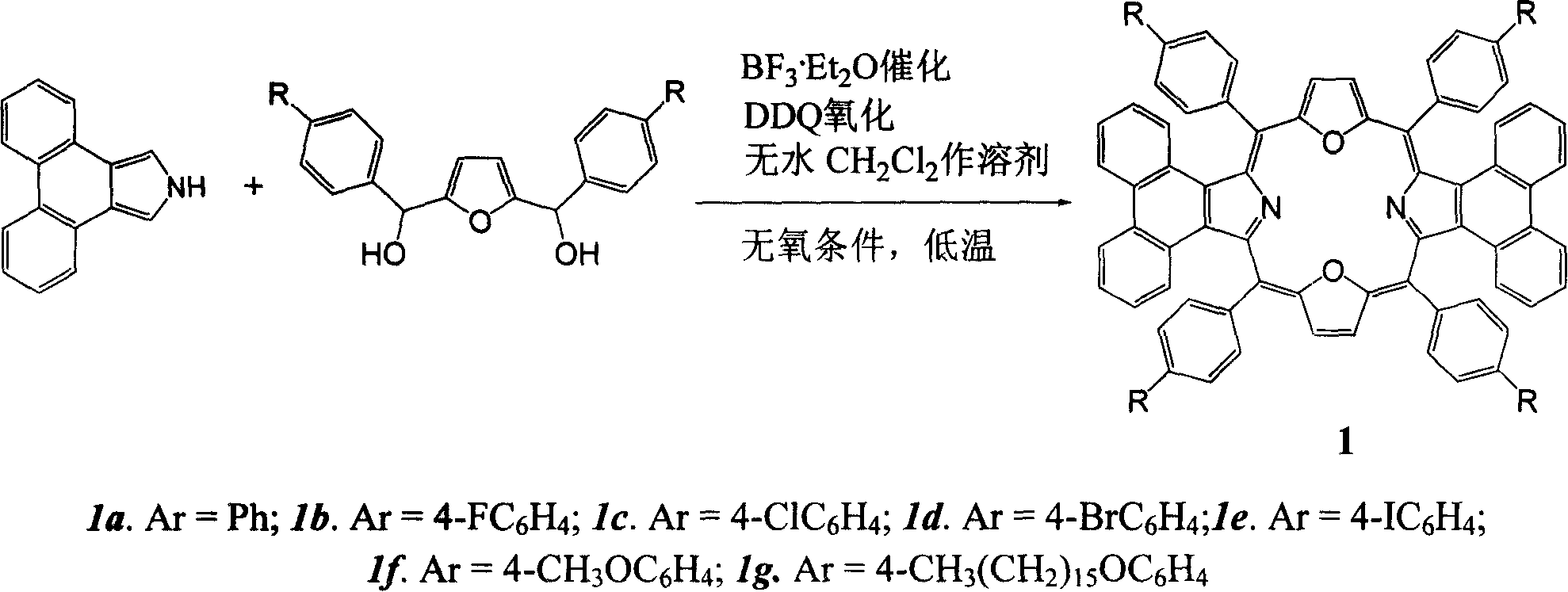
Meso position-tetra phenyl tetra phenanthro porphyrin derivetive and its preparation method
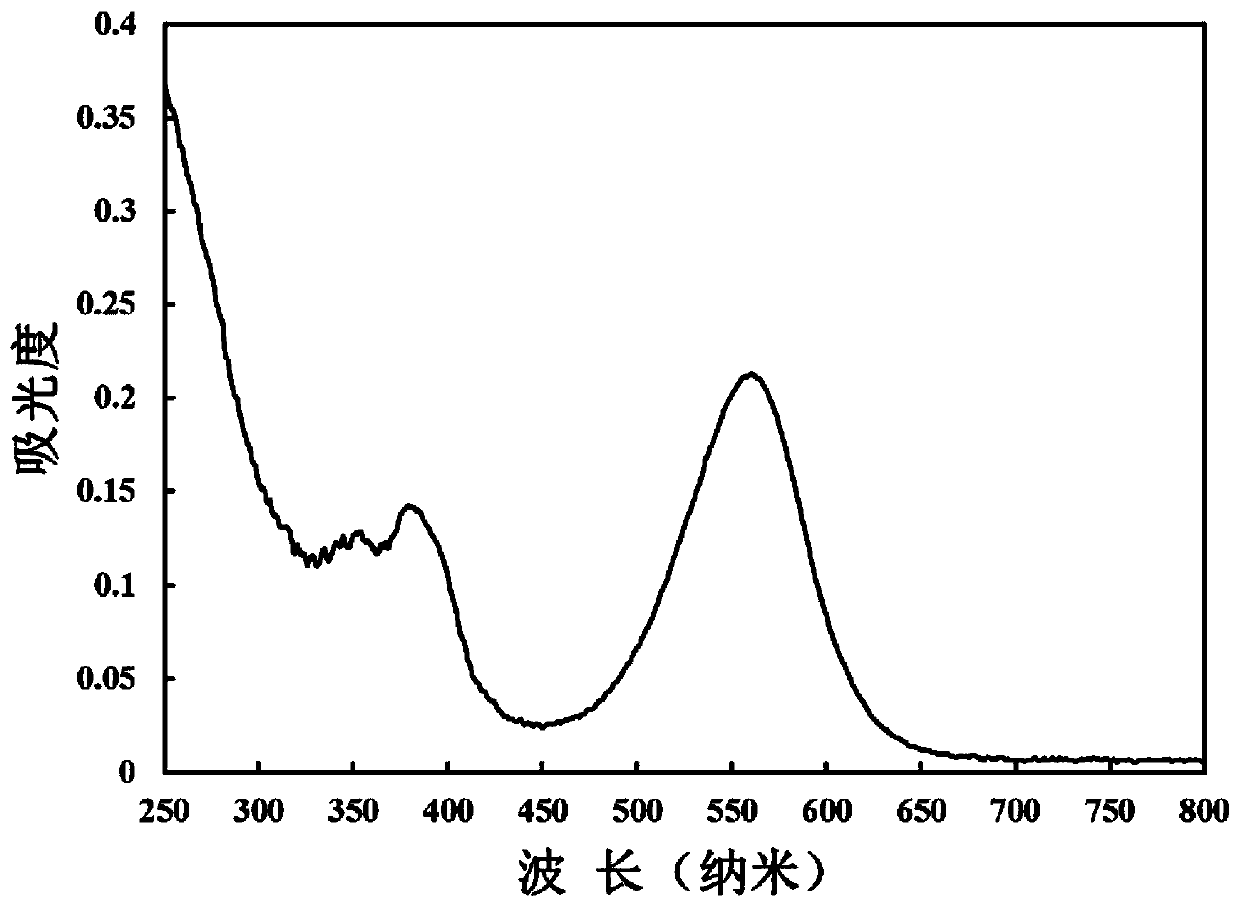
The present invention discloses tetraphenanthryl porphyrin derivative with different substituents in the mid position benzene ring and prepared through the BF3.Et2O catalyzed reaction of phenanthryl pyrrole and benzaldedyde with with different substituents in the para position at low temperature and in no-oxygen atmosphere. These conjugated porphyrin compounds have Soret band in 577-603 nm, red shifted by over 160 nm compared with that of no-aromatic ring conjugated beta-tetraphenyl porphyrin and red shifted by near 100 nm compared with that of phenanthrene ring conjugated porphyrin without substituent in the mid position. These conjugated porphyrin compounds have wide application foreground in OFETs, molecular antenna, light-to-energy converter, medicine and other fields.
Owner:NANJING UNIV
Synthesis and application of (D-A)n+1D type oligomer photovoltaic donor material based on benzodithiophene-4,8-dione
InactiveCN108250222AHigh molar absorptivityPromote absorptionOrganic chemistrySolid-state devicesHeterojunctionOrganic solar cell
The invention relates to a benzo[1,2-c,4,5-c']dithiophene-4,8-dione (BDD)-based electron acceptor, synthesis of an oligomer photovoltaic donor material with a (D-A)n+1D (n is equal to 1,2. . .) framework as well as application of the oligomer photovoltaic donor material to an organic solar battery. The oligomer comprises thiophene, benzene, benzodithiophene and a derivative electron donating (D) unit as well as BDD and a derivative electron acceptor (A) unit, and can serve as a donor material widely applied to a solution processing type organic solar battery. When a light active layer donor material is 5 BDTBDD, an acceptor material is 3,9-bis(2-methylene-3-(1,1-dicyanomethyl)indolone)-5,5,11,11-tetra(4-hexylphenyl)- dithiolato[2,3d,2',3'd]-s-indole[1,2-b,5,6']dithiophene (ITIC), and the highest energy conversion efficiency of bulk heterojunction oligomer solar energy is 7.89 percent when the mass ratio of the donor to the acceptor is 1,0.8. The organic solar battery is constructed bytaking the oligomer as the electron donor material and non-fullerene as the electron acceptor material, and efficient energy conversion of the device is realized.
Owner:CHANGZHOU UNIV
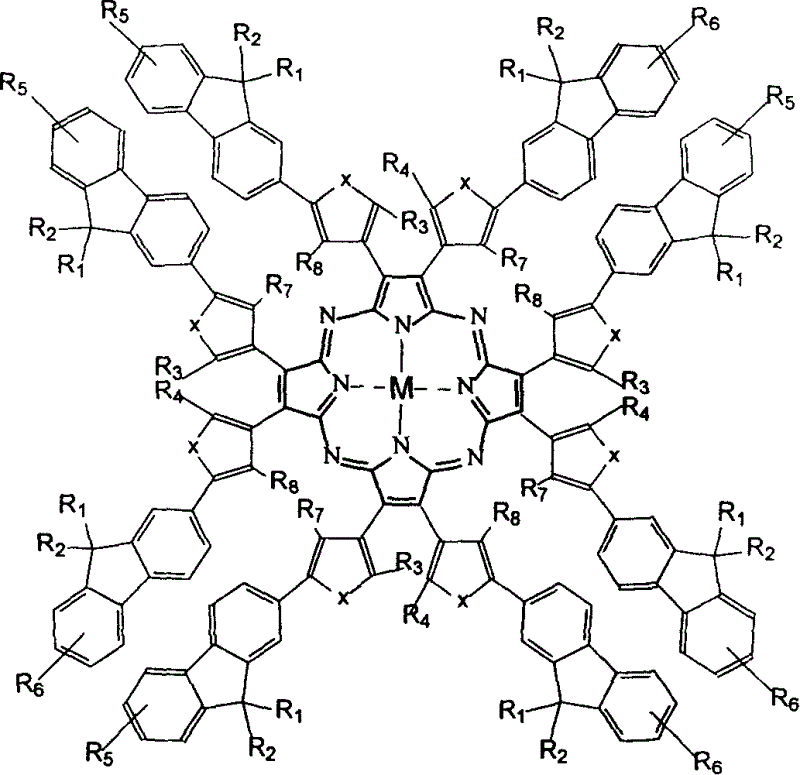
Metal aza porphyrin compound containing fluorene diaryl ethylene and its preparation method and application
InactiveCN1724534AEasy to synthesizeUnique structureTin organic compoundsGroup 8/9/10/18 element organic compoundsSolubilityWavelength
The invention discloses a fluorine-containing diarylethylene metal aza porphyrin compound, its synthesizing method and application, wherein the compound has a rather large molar absorption coefficient and strong near-infrared fluorescence-emission, the charge of alkyl fluorine can increase the solubility of the material in organic solvent and its light-emitting performance. By employing the opening / closing ring reaction of the dithiofuran ethylene, the fluorescent change of the compound can be adjusted, and the non-destructive reading out can be achieved.
Owner:方圆环球光电技术盐城有限公司
Dithiophene pyrrole bridge-indoline organic dyes as well as preparation method and application thereof
InactiveCN103554958AEffective printingHigh molar absorptivityLight-sensitive devicesOrganic chemistryOrganic dyePyrrole
The invention discloses dithiophene pyrrole bridge-indoline organic dyes with the following structural formula which is shown in the specification, wherein R1 is C1-C6 alkoxy, a structural formula of Ar is (II) or (III); in the formula (II) and (III) which are shown in the specification, R2 is C1-C6 alkyls. A preparation method of the dithiophene pyrrole bridge-indoline organic dyes comprises the following steps of: using indoline boron ester, tetra triphenylphosphine, potassium carbonate and dithiophene pyrrole bromal substituted by alkoxy triphenylamine as materials; and obtaining a target through Suzuki coupling reaction and Knoevenagel condensation reaction in sequence, wherein the target can be used as a photosensitizer of a dye-sensitization solar cell. The preparation method disclosed by the invention has the advantage that a molar absorption coefficient and absorbing ability to light of dye molecules can be effectively improved by introducing the dithiophene pyrrole bridge into the indoline dyes; printed electrons can be effectively compounded and open-circuit voltage can be effectively improved by introducing large steric-hindrance groups into the indoline and the dithiophene pyrrole bridge, so that photoelectric conversion efficiency of the solar cell is improved.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Ring-fused structural near-infrared photosensitizer and preparation method thereof
InactiveCN105085556AGood light stabilityHigh molar absorptivityEnergy modified materialsGroup 3/13 element organic compoundsSinglet oxygenBODIPY
The invention relates to a kind of ring-fused structural near-infrared photosensitizer and a preparation method thereof, especially relates to a kind of a photosensitizer treating malignant tumor through photodynamic therapy and a preparation method thereof, and concretely provides a kind of a heavy-atom-substituted near-infrared aza-BODIPY-like (aza-boron dipyrromethene-like) photosensitizer, a preparation method thereof, and efficiency of the photosensitizer generating singlet oxygen. The photosensitizer is a series of novel photosensitizers taking heavy-atom-substituted near-infrared fluorescent dye aza-BODIPY developed from azodipyrromethane boron trifluoride as a mother nucleus. The general formula of the phoosensitizer is shown as formulas I / II / III / IV. The aza-BODIPY-like photosensitizer possesses a ring-fused structure, and also possesses advantages of classic traditional dye BODIPY / aza-BODIPOY spectrum performances. The ring-fused structural aza-BODIPY-like photosensitizer has the absorption wavelength more than 700 nm, is capable of deeply penetrating biological issue under irradiation of a long-wavelength monochromatic light source, possesses low toxicity and low side effect, and is high in singlet oxygen generation efficiency, and electron of the photosensitizer is easy for intersystem crossing.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Pyrrolidine double-fluorine-boron strong fluorescent dye and preparation method and application thereof
ActiveCN108516985ARaw materials are easy to obtainSimple preparation stepsHydrazone dyesGroup 3/13 element organic compoundsQuantum yieldXylylene
The invention discloses a pyrrolidine double-fluorine-boron strong fluorescent dye and a preparation method and application thereof, wherein the structure is as shown in formula (1), R1, R2 and R3 areH or C1-C6 linear or branched alkyl group, R5 is H, Cl, xylylene group, nonane group, benzene ring group, OR9, NR9R10, or SR9. R4, R6, R7 and R8 are each independently H, C1-C6 linear or branched alkyl, C1-C6 linear or branched cycloalkane group; wherein R9 and R10 are each independently H, CH2COOEt, oxo group, xylyl group, C1-C6 linear or branched alkyl or C1-6 linear or branched cycloalkane group; the pyrrolidine double-fluorine-boron strong fluorescent dye has the advantages of up to 998 GM double-photon absorption cross section at 700-900nm, high molar absorption coefficient, high fluorescence quantum yield, high light stability and double photon fluorescence and the like.
Owner:ANHUI NORMAL UNIV
Monosubstituted metal phthalocyanine and synthesis and separation method and application thereof
InactiveCN102070641AIncrease selective uptakeUNIQUE AND EXCELLENT PERFORMANCEOrganic active ingredientsOrganic chemistryOrganic basePhthalonitrile
The invention discloses monosubstituted metal phthalocyanine and a synthesis and separation method and application thereof. The conventional synthesis and separation of a metal phthalocyanine coordination compound are limited by experimental conditions and are not suitable to be widely applied. The monosubstituted metal phthalocyanine is prepared by the following steps of: reacting substituted phthalonitrile 1 with substituted phthalonitrile 3 in the presence of n-amyl alcohol, DMF (N,N-dimetbylformamide) or DMSO (dimethyl sulphoxide) according to a molar ratio; adding metal salt and organic base, wherein the molar ratio of the metal salt to the substituted phthalonitrile 1 is 1:1-1:5, the molar ratio of the organic base to the substituted phthalonitrile 1 is 3:1-10:1, the molar ratio of the n-amyl alcohol, the DMF or the DMSO to the substituted phthalonitrile is 10:1-20:1 and the reaction is performed at the temperature of between 120 and 200 DEG C for 15 to 30 hours to generate a monosubstituted metal phthalocyanine-containing mixture; and separating the mixture by a dry-column chromatography. The method is used for preparing new monosubstituted metal phthalocyanine.
Owner:HEILONGJIANG UNIV
Lead ion detection test paper and preparation method thereof
InactiveCN102643917AHigh sensitivityHigh molar absorptivityMicrobiological testing/measurementIonChemistry
The invention discloses a lead ion detection test paper and a preparation method thereof. The test paper consists of a bar-type base plate, a sample pad, a glass fiber combination pad, a specifically enveloped nitrocellulose membrane and a water absorption pad, wherein the sample pad, the glass fiber combination pad, the specifically enveloped nitrocellulose membrane and the water absorption pad are lapped on the bar-type base plate in sequence; the glass fiber combination pad is coated with a special bar-type code DNA-nano gold-deoxyribozyme probe; the nitrocellulose membrane is provided with a quality control line C and a detection line T; and pairing can be formed between a basic group capturing DNA and bar-type code DNA basic group on the surface of the special bar-type code DNA-nano gold-deoxyribozyme probe. The preparation method comprises the following steps: the prepared sample pad, the glass fiber combination pad coated with the special bar-type code DNA-nano gold-deoxyribozyme probe, the specifically enveloped nitrocellulose membrane and the water absorption pad are lapped, are pasted on the base plate and are cut into a thin strip with the width of 3-5mm to obtain the lead ion detection test paper. The lead ion detection test paper has the advantage of capability of detecting the lead ion in the detection sample in high flexibility and high selectivity.
Owner:NINGBO UNIV
Novel organic dye and method for preparing same
InactiveCN102076781ALower synthesis costImprove efficiencyMethine/polymethine dyesElectrolytic capacitorsOrganic dyePhotocurrent
Owner:DONGJIN SEMICHEM CO LTD
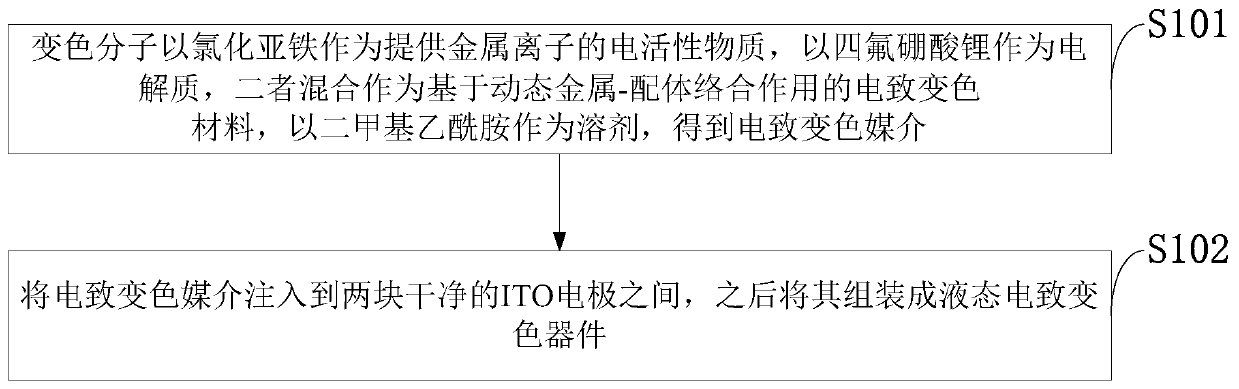
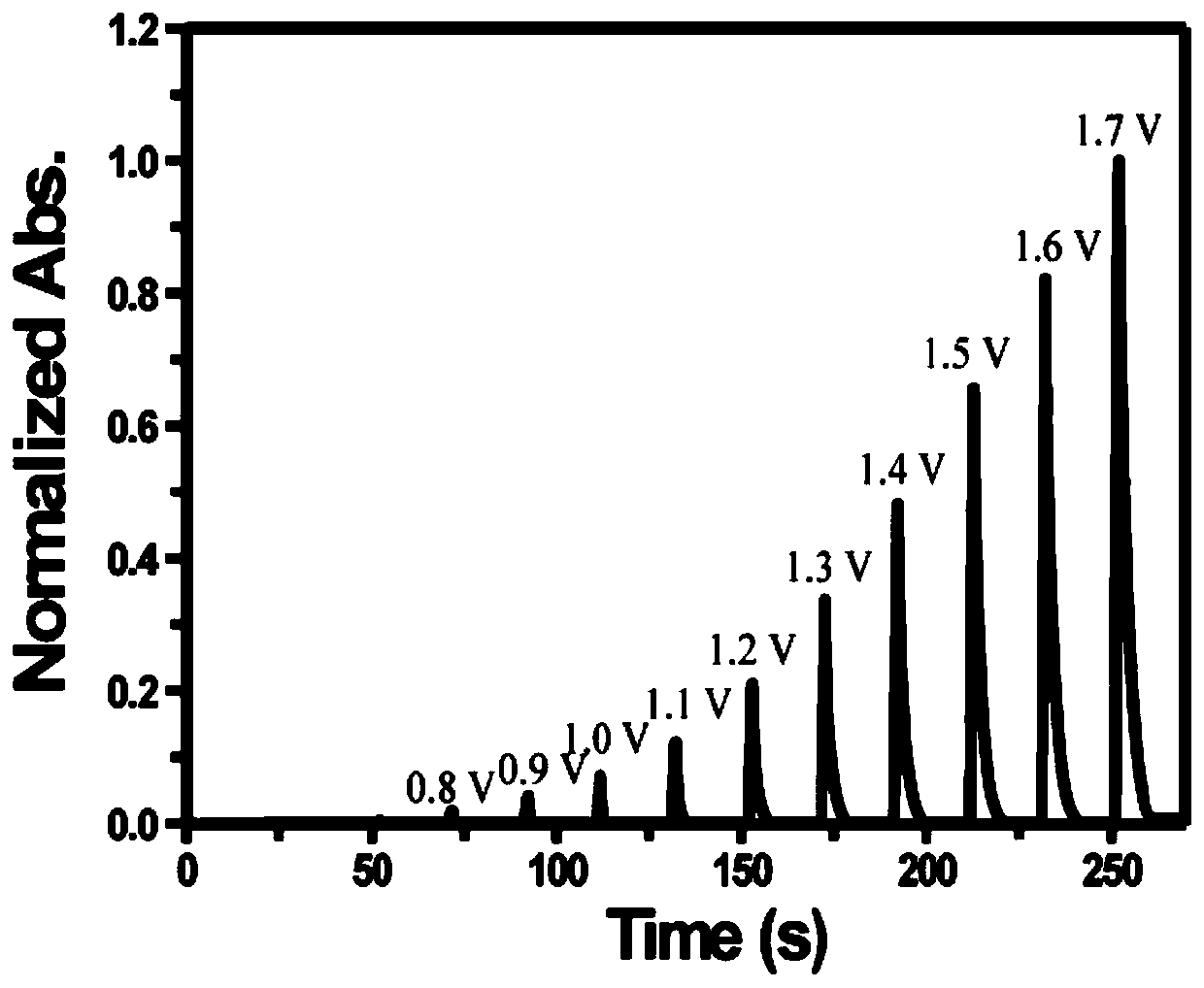
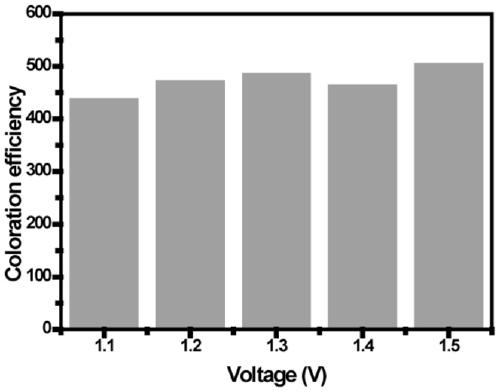
Electrochromic material and electrochromic device based on dynamic metal-ligand complexation
PendingCN111117594AHigh Color Changing ContrastObvious discolorationCopper organic compoundsTenebresent compositionsIon-exchange membranesElectrochromism
The invention belongs to the technical field of electrochromic materials, and discloses an electrochromic material and an electrochromic device based on dynamic metal-ligand complexation. The electrochromic material is formed by mixing metal ions, the electrochromic material and an electrolyte, the electrochromic device is designed based on the dynamic metal-ligand complexation, and the structureof the electrochromic device is divided into a liquid electrochromic device structure and a solid electrochromic device structure. The liquid electrochromic device structurally comprises a first electrode, an electrochromic medium and a second electrode. The solid electrochromic device structurally comprises a first electrode, an electrochromic medium, an ion exchange membrane, an auxiliary mediumand a second electrode. The electrochromic material and the electrochromic device realize adjustable full-visible spectrum and high contrast, and have the advantages of high coloring efficiency, quick electrochromic response, excellent cycle reversibility, ultralow cost and simple preparation process.
Owner:JILIN UNIV
Conjugated polymer containing cyclopentadienyl diene dithiophene-naphthalene tetracarboxylic diimide and preparation method and application thereof
InactiveCN102329414AQuality improvementThe synthetic route is simpleSolid-state devicesSemiconductor/solid-state device manufacturingPolymer scienceHydrogen
The invention discloses a conjugated polymer containing cyclopentadienyl diene dithiophene-naphthalene tetracarboxylic diimide, which is characterized by being a compound with the formula shown as below, wherein R1 is C1-C20 alkyl, R2 is C1-C20 alkyl or substituted phenyl, R3, R4 and R5 are identically or differently expressed as hydrogen, C1-C20 alkyl or alkoxy, x is greater than or equal to 1 and less than 2, y is greater than 0 and less than or equal to 1, the sum of x and y is 2 and n is an integer from 1 to 120. The conjugated polymer containing cyclopentadienyl diene dithiophene-naphthalene tetracarboxylic diimide is prepared via Stille with a simple reactive synthesis route and low processing requirements and has better film-forming properties, better thermal stability, high carrier mobility, higher molar absorption coefficient and wide optical absorption range, the degree of matching with solar transmission spectrum is improved, the photoelectric conversion efficiency is increased, and the conjugated polymer has a wide scope of application.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +1
Thioxanthone acetylfluorene photoinitiator and preparation method thereof
InactiveCN104371043AEasy to prepareConditions are easy to controlOrganic chemistrySynthesis methodsThiosalicylic acid
The invention discloses a thioxanthone acetylfluorene photoinitiator and a preparation method thereof. The preparation method comprises the following steps: adding a solvent, thiosalicylic acid and 2-acetylfluorene in a reactor, reacting under the condition of heating until the reaction is finished; and cooling reaction liquid obtained after reaction, carrying out aftertreatment including washing and recrystallizing to obtain thioxanthone acetylfluorene. Under the same light condition, the thioxanthone photoinitiator with larger molar absorption coefficient has stronger photo-initiation function. The synthesis method of the thioxanthone acetylfluorene photoinitiator is simple; the thioxanthone acetylfluorene photoinitiator has an absorption platform in a near ultraviolet light region, and has a molar absorption coefficient of 7983L.mol<-1>.cm<-1> at the wavelength of 398nm; as an efficient near ultraviolet photoinitiator, the thioxanthone acetylfluorene photoinitiator can be capable of greatly improving the photo-initiation efficiency and has the beneficial effects of conservation of energy sources and improvement of the output and quality of related products.
Owner:JINYU HENAN PACKAGING
Semi-sandwich iridium complex having fluorescent property and containing N-N two-tooth chelated ligand, and preparation method and application of semi-sandwich iridium complex
ActiveCN108276454AHigh anticancer activityHigh fluorescence quantum yieldOrganic active ingredientsPharmaceutical non-active ingredientsVisibilityIridium
The invention relates to a semi-sandwich iridium complex having fluorescent properties and containing N-N two-tooth chelated ligand and belongs to the field of chemical pharmaceutical. The molecular structural formula of the iridium complex is as shown in the specification. As the iridium complex is creatively modified with rhodamine B, the whole complex has high anti-cancer activity and mitochondria targeting, has selectivity upon cancer cells, action process visibility and ideal purposes of real-time detection, and has a great significance on medicine targeting research. N-N is adopted as anion ligand for two-tooth chelation, the novel anionic iridium complex with relatively high anti-cancer activity and fluorescent properties can be synthesized, and the complex is good in effect and high in activity in cancer prevention and cell imaging.
Owner:QUFU NORMAL UNIV
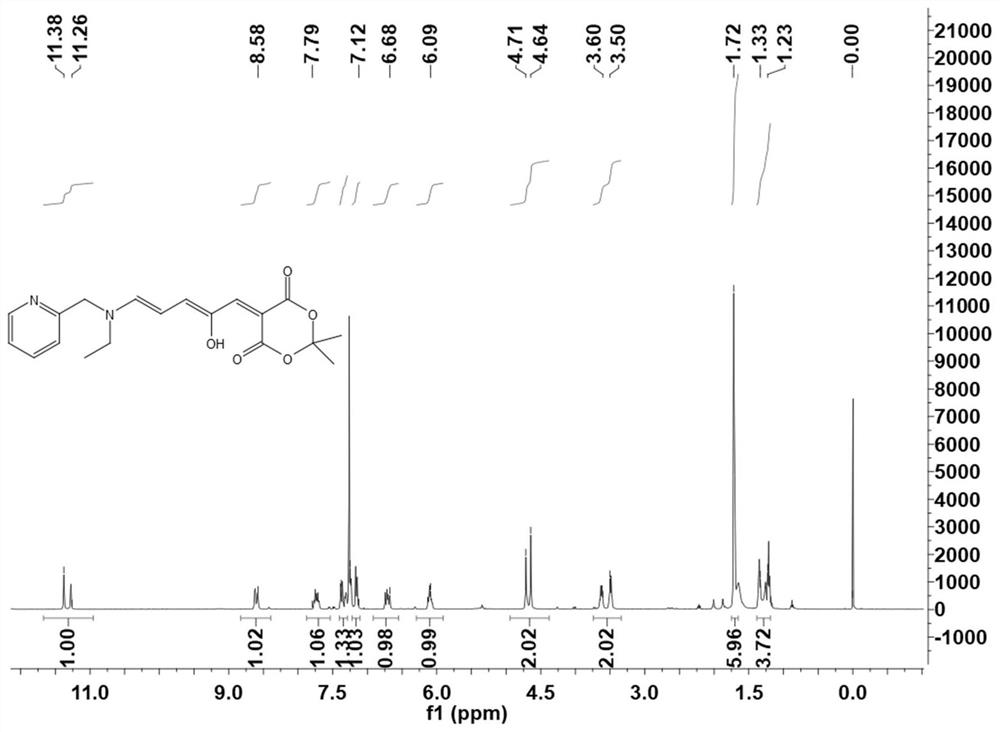
Stenhouse donor-receptor adduct of Meldrum's acid-activated furan and 3-pyridylethylamine, and synthesis method thereof
The invention discloses a Stenhouse donor-receptor adduct (DASA) based on Meldrum's acid activated furan and 3-pyridylethylamine, and a synthesis method of the Stenhouse donor-receptor adduct. The method specifically comprises the following steps: mixing 2-furan formaldehyde and cycloisopropyl malonate to react to synthesize an intermediate, and reacting the intermediate with 3-pyridylethylamine to generate a DASA compound. According to the method, the organic solvent with low toxicity is used for carrying out addition reaction under certain conditions to obtain the DASA compound with high yield and high content, and a catalyst is not needed, so that the production cost is effectively reduced; and the photophysical property test shows that the compound has different performances in different solvents, which indicates that the compound has a wide application range, can satisfy different demands in different fields, and fully exerts the excellent performances of the compound.
Owner:NANJING UNIV OF SCI & TECH
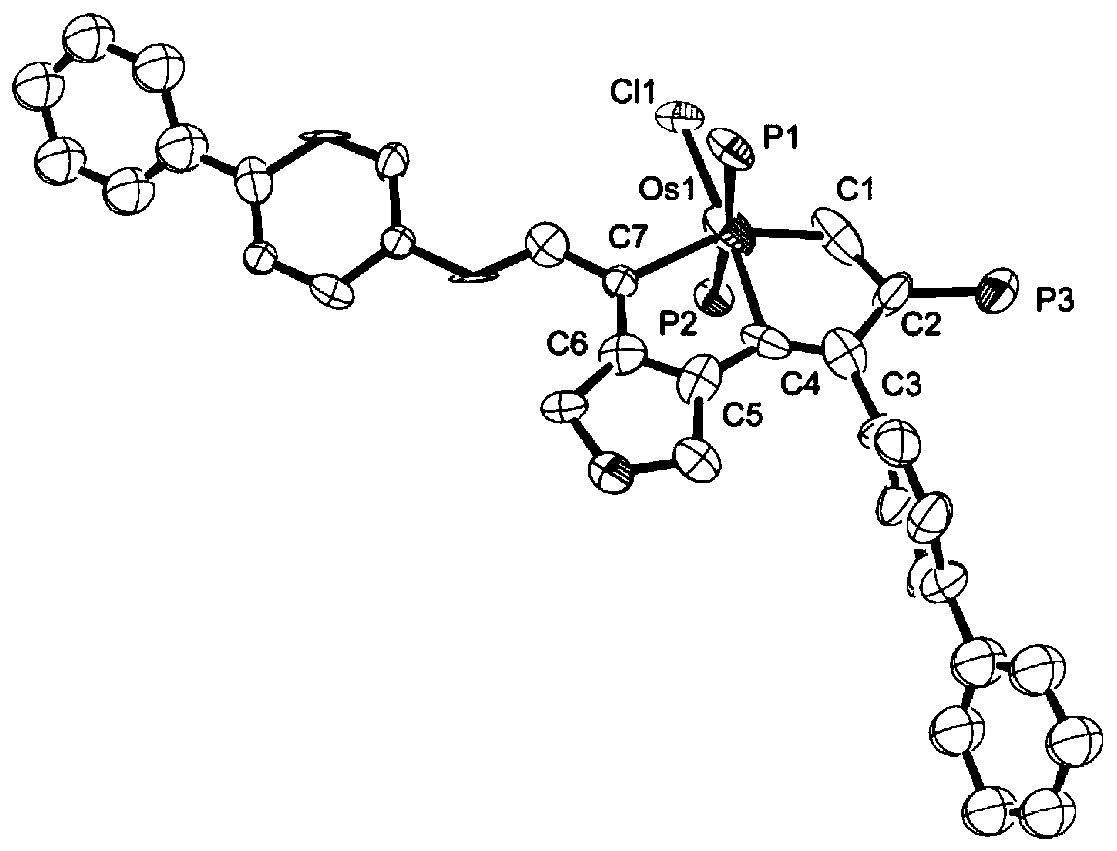
Preparation method and application of 7-position vinyl substituted osmapentalyne
ActiveCN110684050AImprove solubilityImprove stabilityOrganic-compounds/hydrides/coordination-complexes catalystsSolid-state devicesOrganic solar cellPhotodynamic therapy
The invention discloses a preparation method of 7-position vinyl substituted osmapentalyne. The reaction formula is as follows: the 7-position vinyl substituted osmapentalyne prepared by the inventionhas high solubility, good stability, very high molar absorption coefficient and better electrochemical performance, and can be widely applied to dye sensitized solar cells, organic solar cells, photodynamic therapy, photolysis of water and lithium-air batteries and other fields. The reaction of the preparation method prepared by the invention has the characteristics of high efficiency and specificity, mild reaction conditions and good functional group tolerance.
Owner:XIAMEN UNIV
Synthesis of middle position-tetraaryldiphenanthrene dioxyporphyrin derivative and application thereof
InactiveCN101210017AImprove absorption efficiencyImprove conversion efficiencyOrganic chemistryLuminescent compositionsMolecular identificationOrganic solar cell
The invention discloses a method for preparing 5,10,15,20-tetra-aryl diphenanthrene (9,10-b;9,10-1)-22,24-diseleno porphyrin compound by the reaction of phenanthro-pyrrole and 2,5-di(aryl hydroxyl methyl) selenophen at low temperature with the function of BF3 Et2O under water free and oxygen free condition. The Soret spectral bands of the compound appear in 521nm, compared with tetraphenyl diseleno porphyrin without conjugated aromatic ring at beta-site, the Soret spectral bands are red shifted by 52nm and enter green light zone above 500nm. The compound has wide application prospects in such areas as photodynamics therapy photosensitizer, OFETs (organic field effect tube), molecular antenna, light energy converter, optical conversion material, molecular switch, molecular logic gate, molecular wire, organic solar battery, organic electroluminescence, non-linear optical material, optical storage, molecular identification and medicine and so on.
Owner:NANJING UNIV
Tripolyindenyl conjugated tri-BODIPY near-infrared fluorescent dye and preparation method thereof
ActiveCN111718365AThe preparation method is simple and easyGood solubilityAzo dyesGroup 3/13 element organic compoundsKnoevenagel condensationFluoProbes
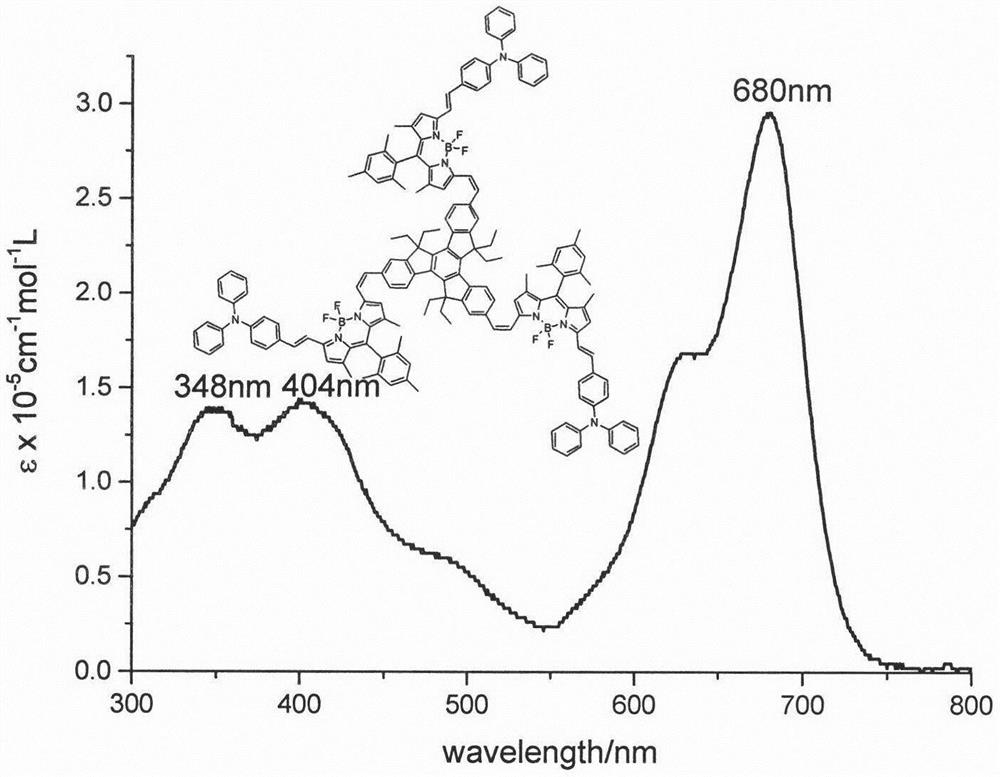
The invention relates to a tripolyindenyl conjugated tri-BODIPY near-infrared fluorescent dye and a preparation method thereof. The tripolyindenyl conjugated tri-BODIPY near-infrared fluorescent dye is synthesized by carrying out Knoevenagel condensation reaction on a BODIPY derivative and tripolyindenyl containing 2, 7, 12-triformyl under the catalytic action of p-toluenesulfonic acid and piperidine. The preparation method has the advantages of simple reaction steps, mild reaction conditions and good selectivity. The fluorescent dye has the advantages of high molar extinction coefficient, favorable solubility, favorable light stability and other excellent photophysical properties. The red shift of the strongest electron absorption spectrum of the dye reaches 650 nm or above, the maximum fluorescence emission wavelength reaches 700 nm or above, and the dye is a near-infrared organic fluorescent dye with a very good application prospect and can be applied to the fields of biomarkers, fluorescent probes, photovoltaic materials and the like.
Owner:NANJING FORESTRY UNIV
Photosensitizer and photoelectric conversion element
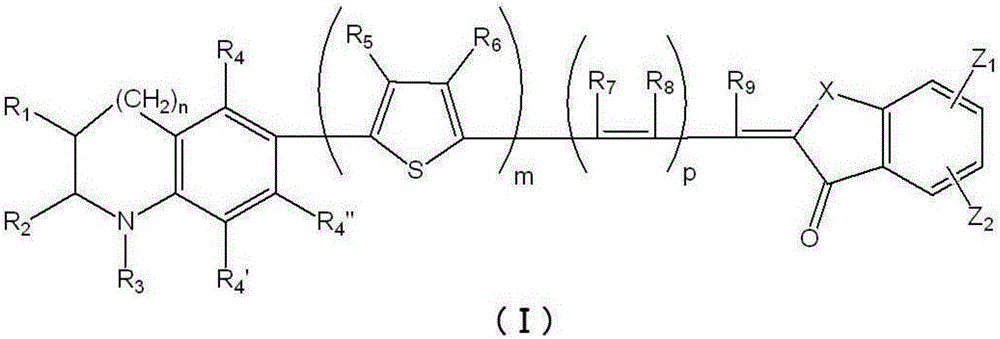
ActiveCN106463272AImprove adsorption stabilityImprove photoelectric conversion efficiencyLight-sensitive devicesMethine/polymethine dyesArylPhotosensitizer
The invention provides a photosensitizer which has excellent conversion efficiency and durability. A photosensitizer which is characterized by being a dye represented by formula (I) or a salt thereof. In the chemical formula, m represents an integer of 0-4; n represents an integer of 0 or 1; p represents an integer of 0-2; each of R1, R2 and R3 represents a hydrogen atom, an alkyl group, an aryl group or the like; each of R4, R4', R4", R5, R6, R7, R8 and R9 represents a hydrogen atom, an alkyl group or the like; X represents a carbonyl group or the like; and each of Z1 and Z2 represents a carboxyl group or the like.
Owner:CHEMICREA
D(pi-A)2 type small molecular donor material based on pyrazine indole terminal receptors, and preparation method and application thereof
ActiveCN110483555AImprove mobilityHigh Photoelectric Efficiency ConversionOrganic chemistrySolid-state devicesHeterojunctionProcessing type
The invention belongs to the field of organic small molecule solar cells, and particularly relates to a D(pi-A)2 type small molecule donor material based on a pyrazine indole terminal electron acceptor and a preparation method and application thereof. An electron donating (D) unit of the D(pi-A)2 type small molecule is 3, 6-bis-(octylthio) thienothiophene (TT), a pi bridge unit is alkylated, oxyalkylated or thioalkylated thiophene or selenophene, an electron withdrawing (A) unit is a novel pyrazine indole derivative, and the small molecule donor material is applied to a solution processing type small molecule solar cell. PC71BM is used as a receptor, and the energy conversion efficiency of the bulk heterojunction solar cell reaches 7.31%. According to the invention, high-efficiency energyconversion of a small molecular donor material constructed based on a pyrazine indole derivative terminal receptor unit in a small molecular solar cell is realized.
Owner:CHANGZHOU UNIV
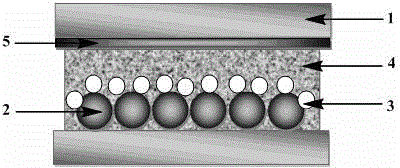
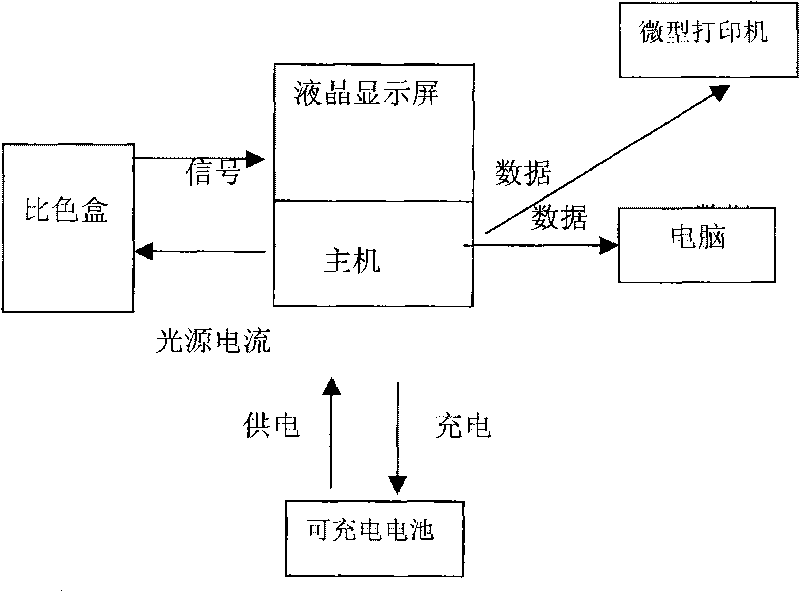
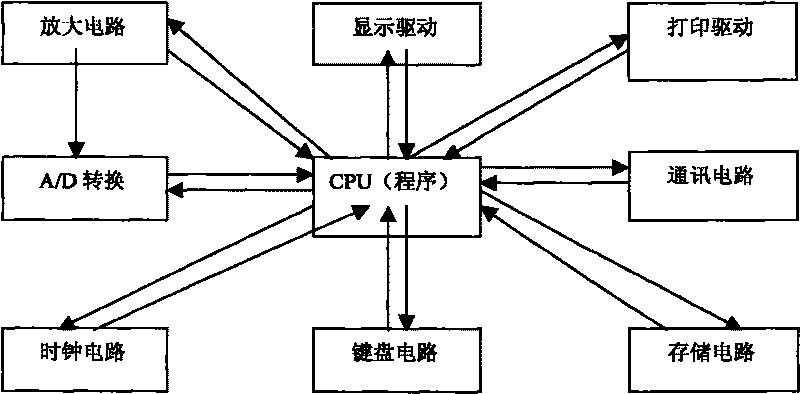
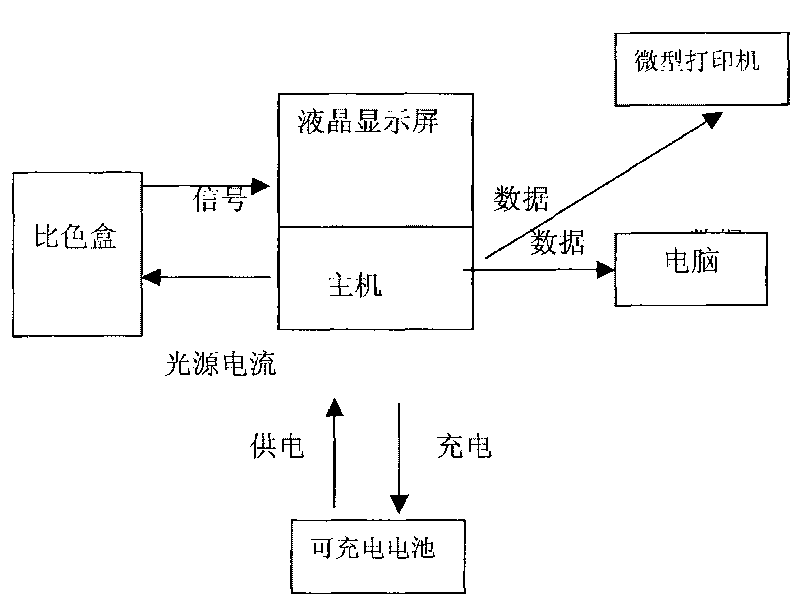
Instrument for rapidly detecting snail-killing agent of niclosamide on site
InactiveCN101718687AExtended Conjugate StructureIncrease the effective area of light absorptionColor/spectral properties measurementsData acquisitionRechargeable cell
The invention relates to an instrument for rapidly detecting the snail-killing agent of niclosamide on site, belonging to the technical field of the instrument for detecting. The instrument for rapidly detecting the snail-killing agent of niclosamide on site comprises a shell. A host, a rechargeable battery and a color comparison box are arranged in the shell. The shell has the functions of controlling the action of the instrument, collecting data, computing, displaying, storing and processing. The color comparison box is a sealed black cubic box, one side of the color comparison box is provided with a photoelectric cell, and the other end is provided with an LED lamp. The rechargeable battery is used for supplying working power to both the host and the photoelectric cell. The instrument can be used for rapidly and conveniently detecting the application dosage of niclosamide ethanolamine salt wettable powder on site.
Owner:扬州市疾病预防控制中心 +2
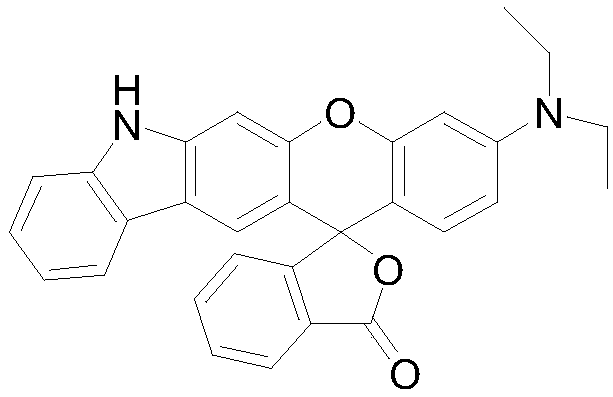
Preparation method and application of fluorescent dye of carbazole-rhodamine hybrid structures
InactiveCN108191880AHigh molar absorptivityHigh fluorescence quantum yieldOrganic chemistryAzo dyesCarbazoleLength wave
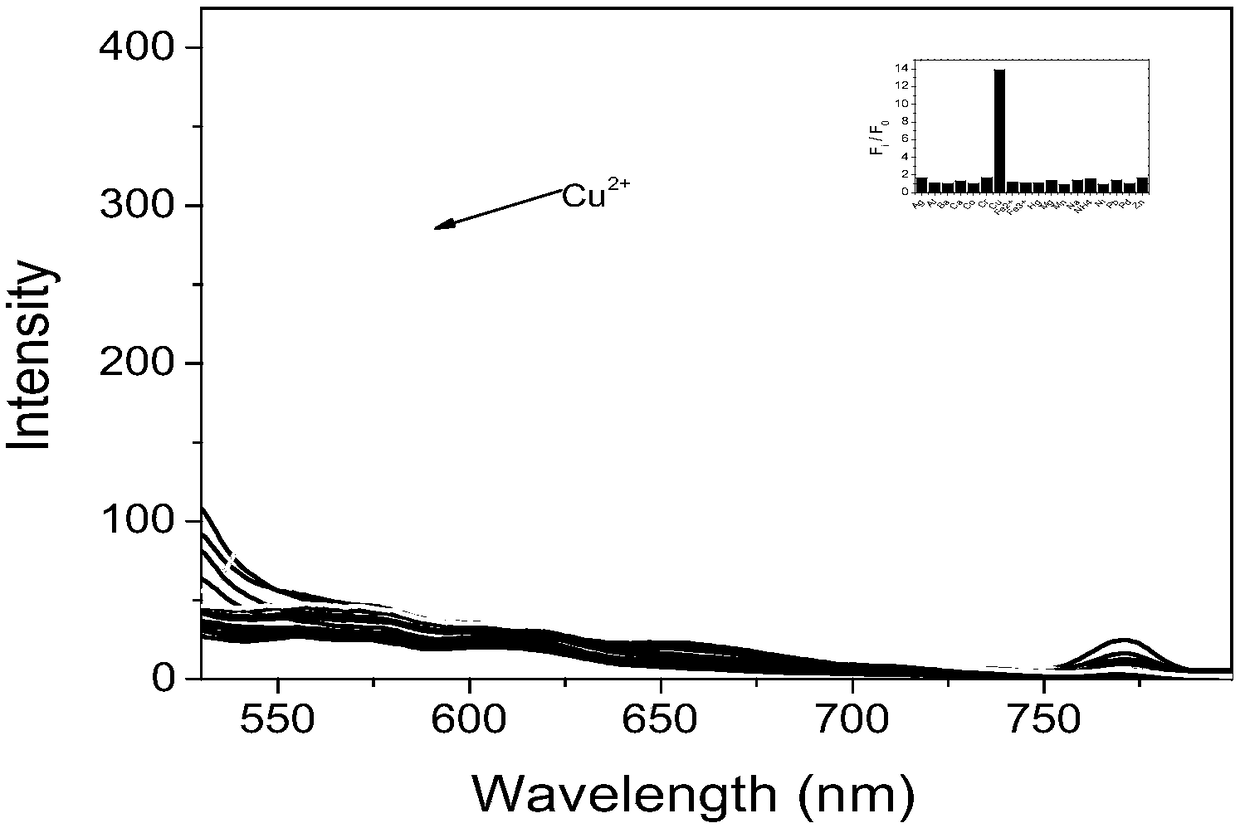
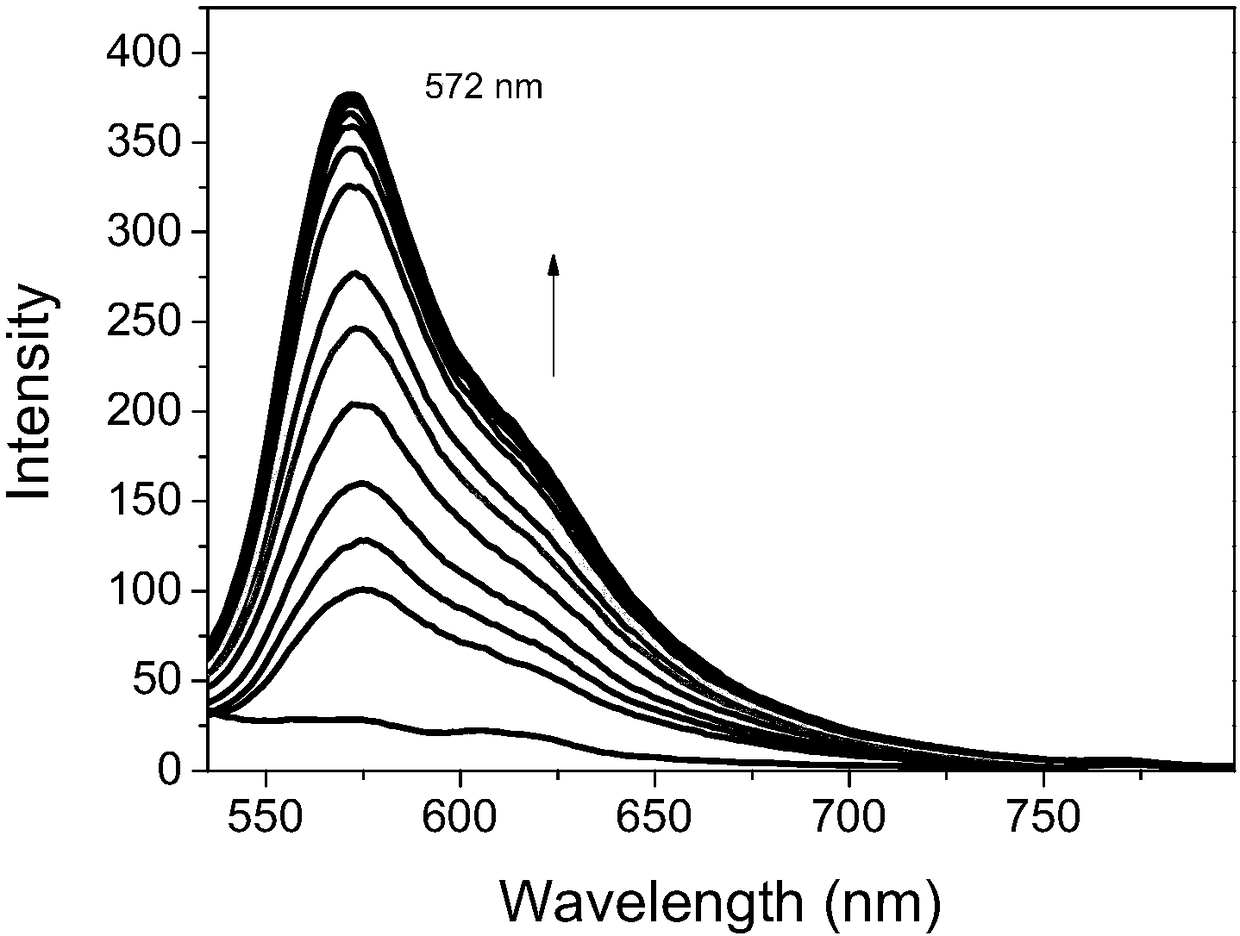
The invention provides a preparation method and application of fluorescent dye of carbazole-rhodamine hybrid structures. The method has the advantages that the designed and synthesized novel carbazole-rhodamine fluorescent dye sufficiently uses the various good properties of carbazole in the field of a photoelectric material as a structure base element and good optical physical and optical chemical properties of rhodamine dye. Through such a hybrid design, the dye shows a greater molar absorption coefficient, longer fluorescence emission wavelength, more flexible and controllable adsorption and fluorescence emission wavelength and the like than the carbazole base element structure. A fluorescence probe designed and synthesized by using the hybridization dye has good selectivity on copper ions; the primary application value of the dye is shown.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Phenylvinyl-BODIPY derivative and preparation method thereof
InactiveCN107903282AEasy to synthesizeEasy to manufactureGroup 3/13 element organic compoundsLuminescent compositionsEthyl propanoateBoric acid
The invention provides a phenylvinyl-BODIPY derivative, the structure of which is as follows: shown in the specification, wherein R1 is alkoxy, Br, boric acid ester, N,N-dimethylamino or diphenylaminegroup, and R is H, 4-bromophenyl / 4-iodobiphenyl or ethyl propionate. Specifically, ten new compounds are provided, and are convenient to synthesize and easy to prepare and purify. The phenylvinyl-BODIPY derivative has extremely high molar absorption coefficient and wide ultraviolet-visible absorption spectrum, and has a wide application prospect on solar batteries and biological detection.
Owner:HUANGHE S & T COLLEGE
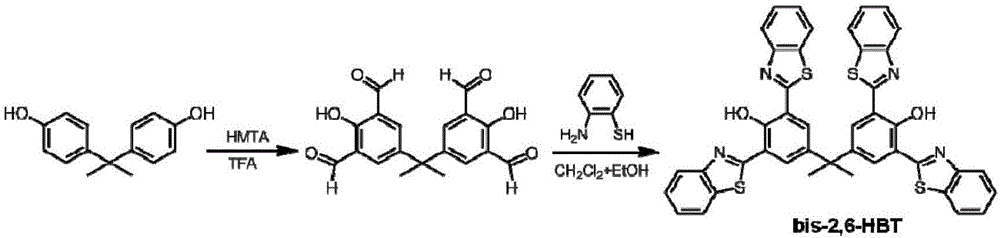
Benzothiazole derivative with optical spectrum signal amplification performance and preparation and application thereof
InactiveCN106749095AHigh molar absorptivityHigh fluorescence quantum yieldOrganic chemistrySolid-state devicesQuantum yield2-Aminothiophenol
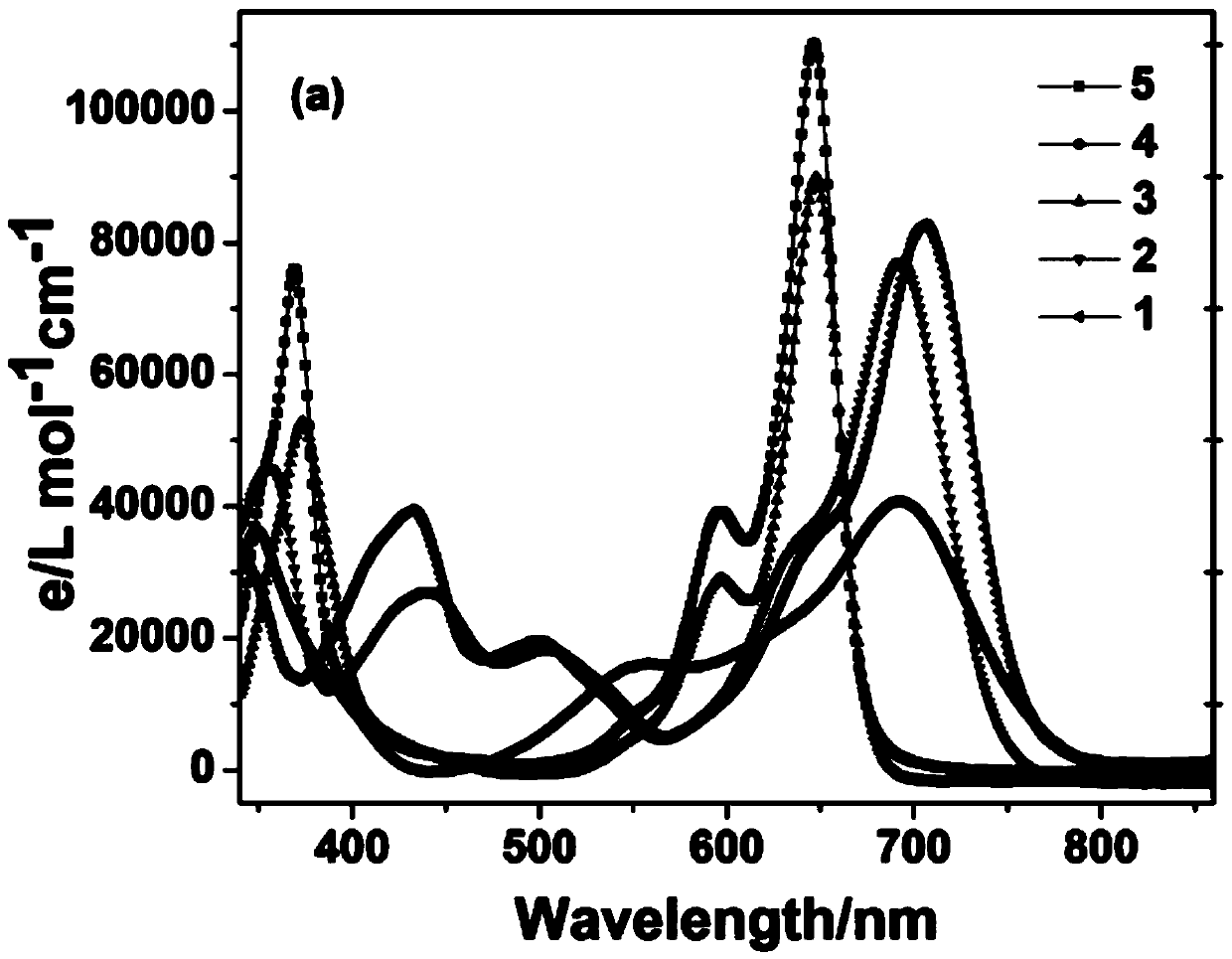
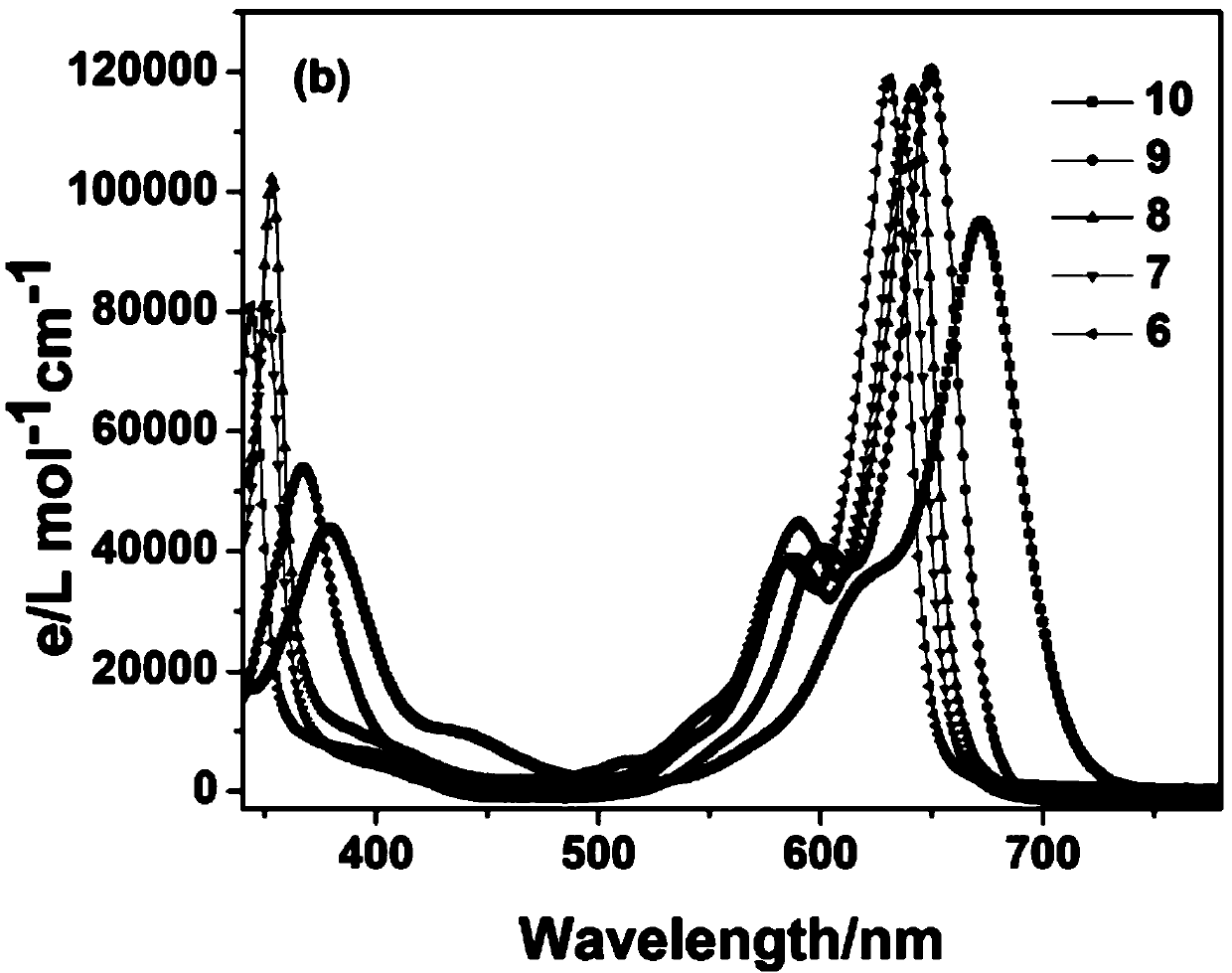
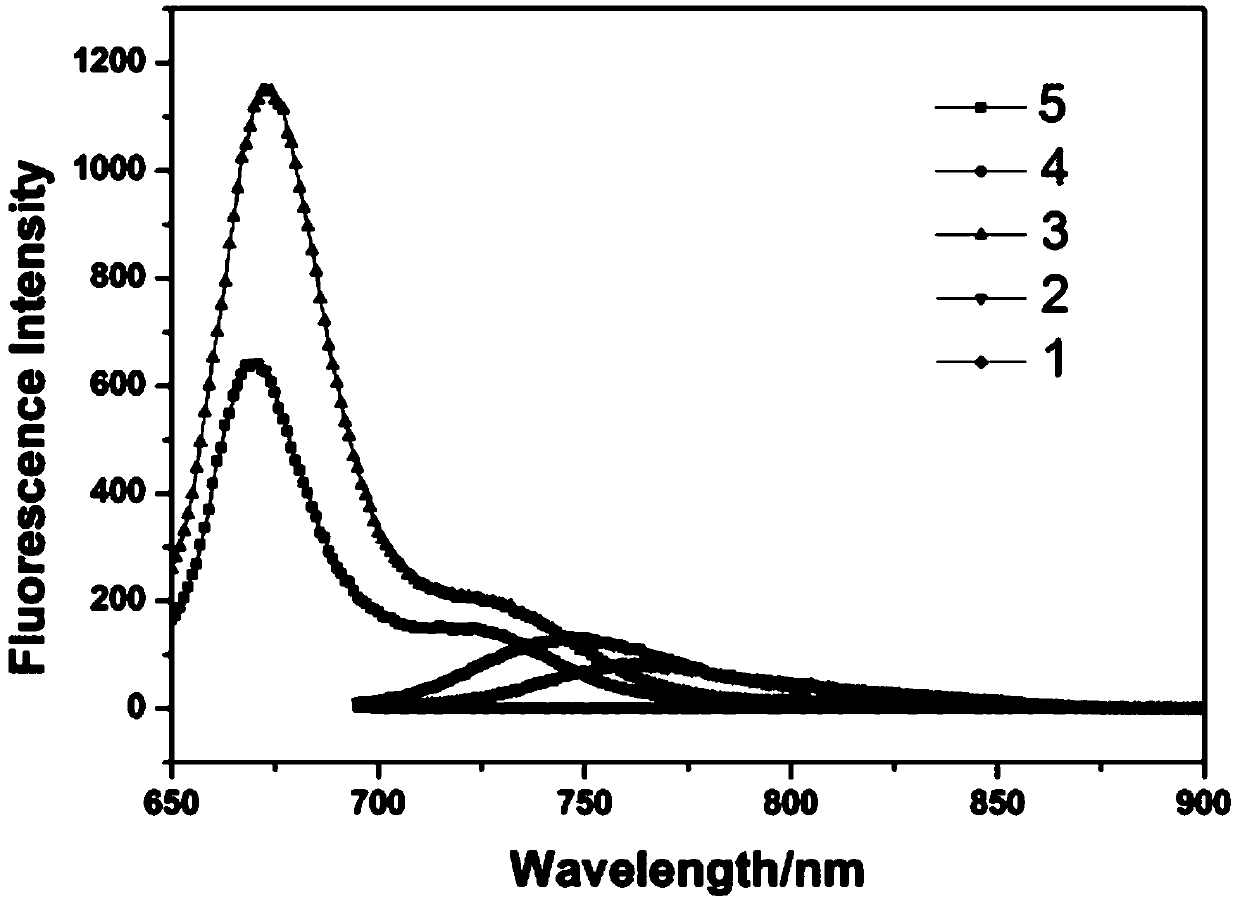
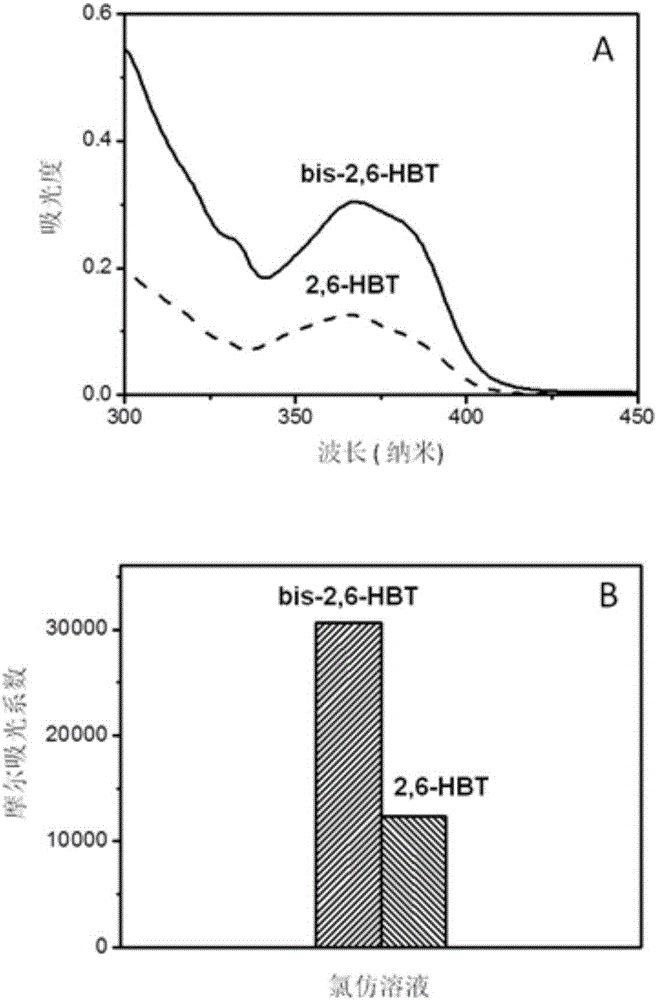
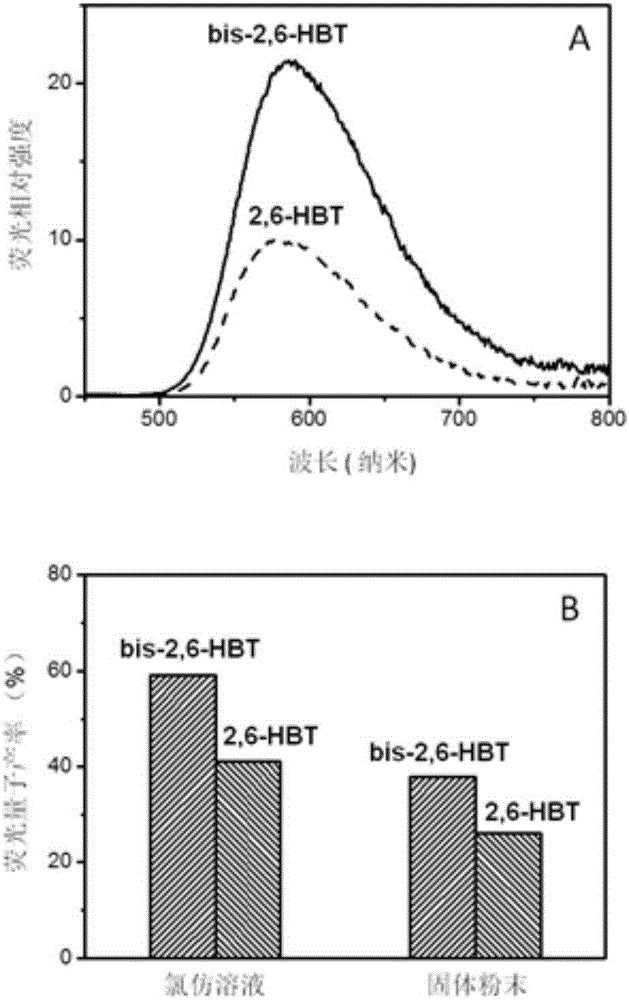
The invention relates to a benzothiazole derivative with optical spectrum signal amplification performance and preparation and application thereof. The benzothiazole derivative has a structural formula shown in the description. The preparation comprises the steps of subjecting bisphenol A tetraaldehyde and 2-aminothiophenol to a reaction at room temperature in a mixed solvent of dichloromethane and ethanol, and carrying out filtering and washing, thereby obtaining the benzothiazole derivative. Both molar absorption coefficient and fluorescent quantum yield of bis-2,6-HBT in a chloroform solution are remarkably increased, and the optical spectrum signal amplification performance is shown. The benzothiazole derivative provided by the invention is simple in preparation method, high in molar absorption coefficient and high in fluorescent quantum yield and has a potential application prospect in the development of light-catching antenna molecules, photosensors and organic luminescent materials.
Owner:DONGHUA UNIV
Furfural-based organic photochromic material and preparation method thereof
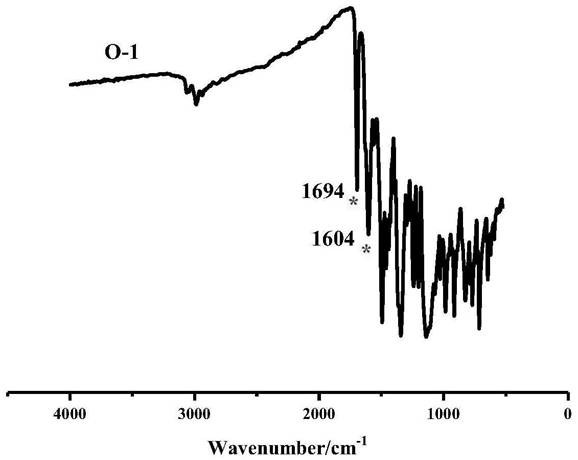
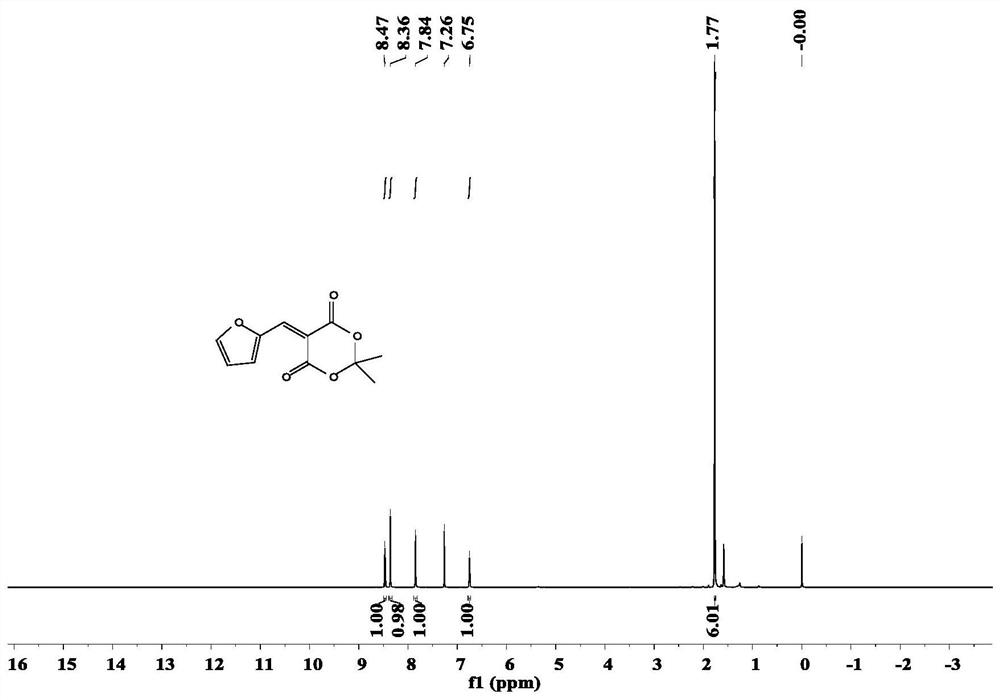
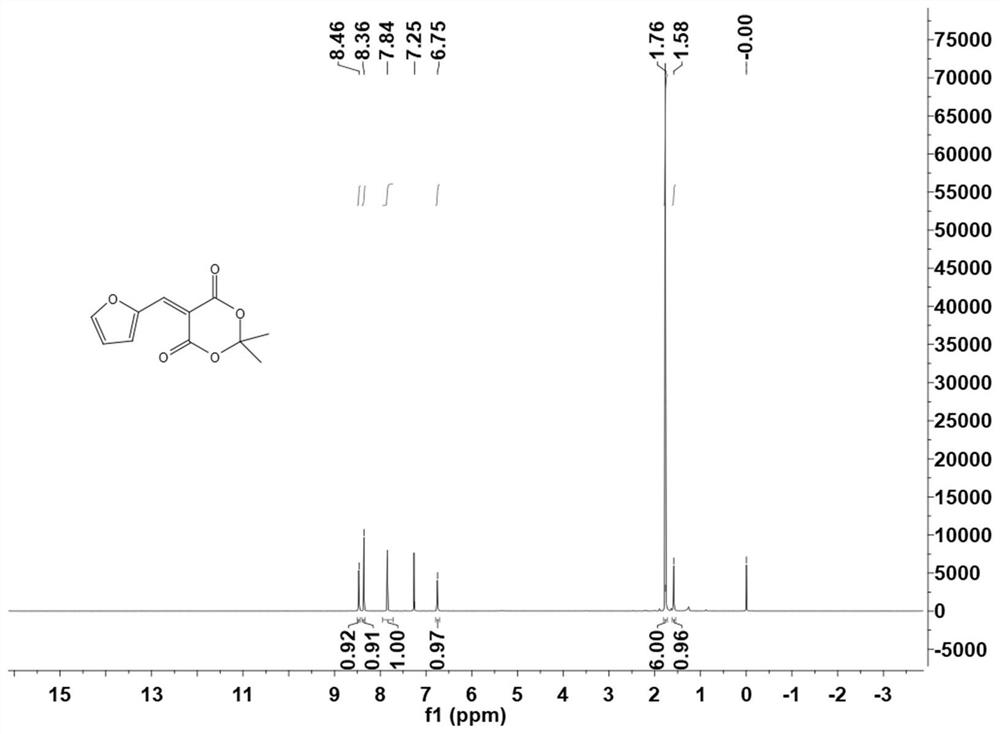
ActiveCN112409342AEasy to getHigh yieldOrganic chemistry methodsTenebresent compositionsPtru catalystFurfural
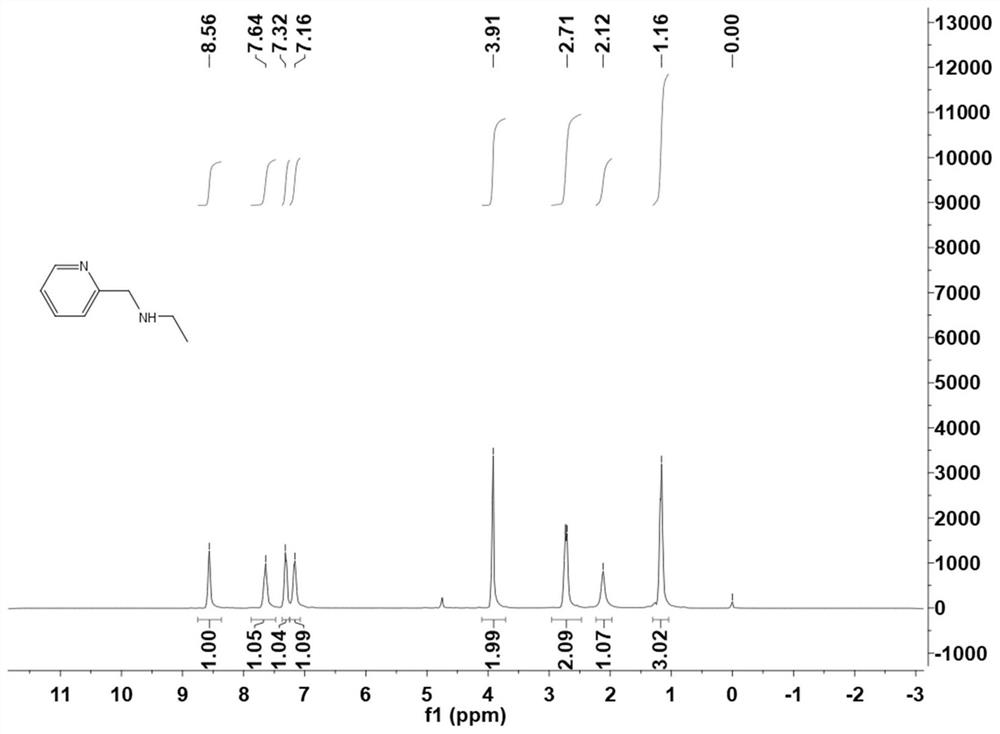
The invention discloses a furfural-based organic photochromic compound and a synthesis method thereof, the method comprises the following steps: reacting malonic acid cyclic (methylene) isopropyl ester with furfural to synthesize a compound I, adding a synthesized compound II, i.e. pyridine-2-ethylamine to generate a target compound III, and recrystallizing in a methanol solution to synthesize anisomer IV. A proper amount of alkali is added into the aqueous solution of the target compound III, and then converted into an isomer V; the organic photochromic compound with high yield and high content is obtained by using an organic solvent with lower toxicity and carrying out condensation reaction under certain conditions, and a catalyst is not needed, so that the production cost is effectively reduced, and the production efficiency is improved. The compound has wide application in the fields of molecular electron and information processing, light-operated catalysis, molecular materials, drug delivery, imaging and control of biological systems and the like.
Owner:NANJING UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com