Pyrrolidine double-fluorine-boron strong fluorescent dye and preparation method and application thereof
A technology of pyrrole pyridine hydrazine and fluorescent dyes, applied in hydrazone dyes, luminescent materials, fluorescence/phosphorescence, etc., can solve problems such as undiscovered two-photon performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
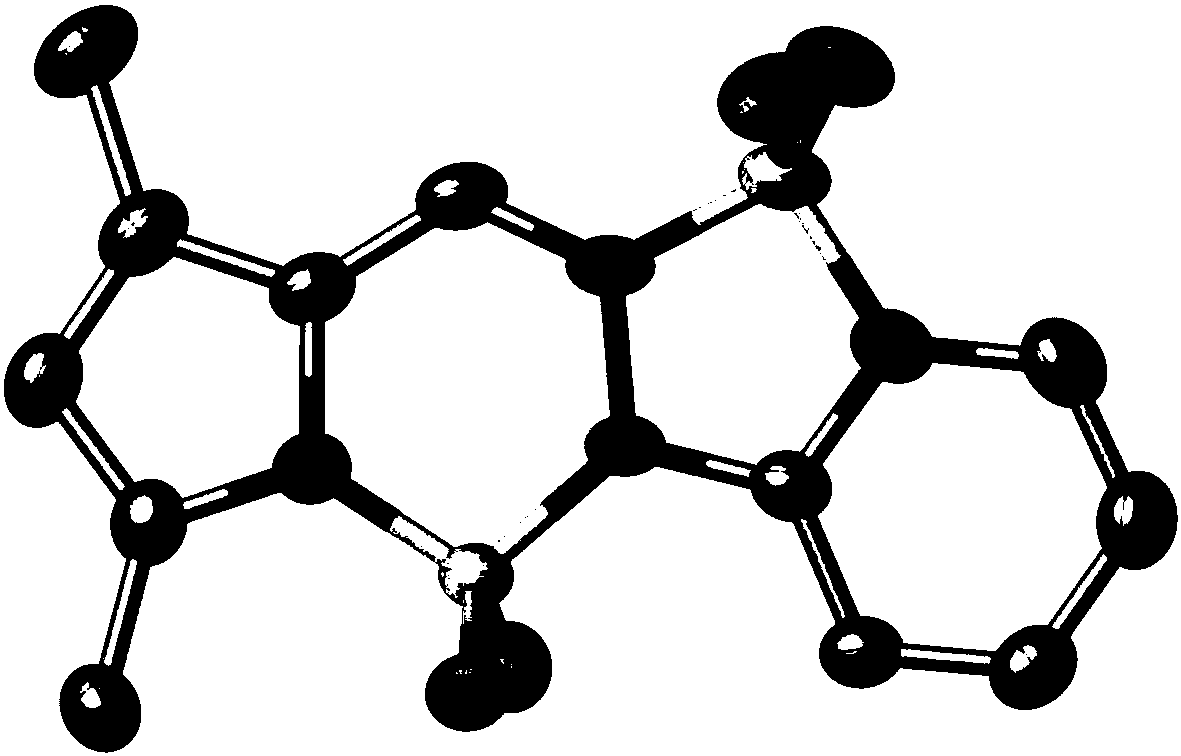
[0052] Synthesis of the strong fluorescent dye 1aa of pyrrole pyridinehydrazine difluoroboron:
[0053]
[0054] 2-Pyrrole aldehyde (190mg, 2mmol) and pyridine hydrazine (230mg, 2.1mmol) were dissolved in 2-dichloroethane (60ml), and p-toluenesulfonic acid (87mg, 0.05mmol) was added. The reaction mixture was heated to reflux for 6 h, followed by TLC spot plate. When the 2-pyrrole aldehyde derivative disappears on the silica gel plate, that is, when the reaction is complete, add 2-10 mL of N,N-diisopropylethylamine into the reaction system. After the reaction mixture was stirred for 10 min, boron trifluoride ether (3-20 ml) was added, and the reaction system was stirred and refluxed for 1-24 h. After cooling to room temperature, the reaction mixture was transferred to a separatory funnel, and dichloromethane and water were added. The organic phase was separated, the corresponding aqueous phase was extracted several times with dichloromethane, and the organic layers were co...
Embodiment example 2
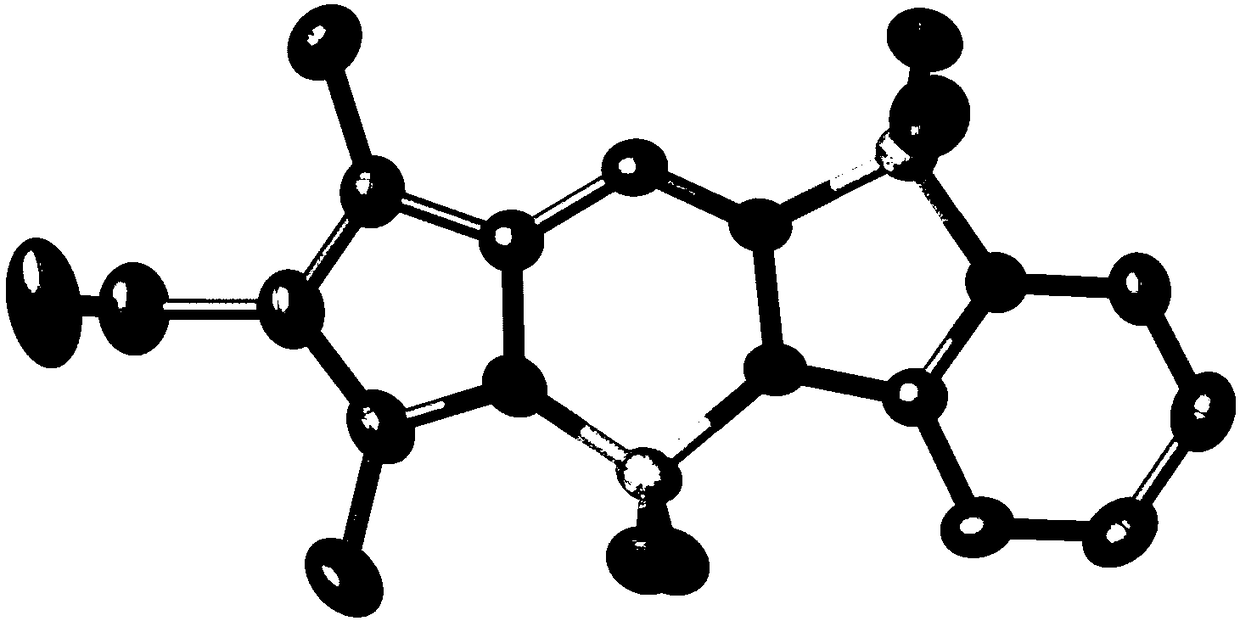
[0057] Synthesis of strong fluorescent dye 1ba from pyrrole pyridinehydrazine difluoroboron:
[0058]
[0059] According to the method of Example 1, the difference is that starting from 2,4-dimethylpyrrole aldehyde (246mg, 2mmol) and pyridine hydrazine (230mg, 2.1mmol), the yield of 1ba was 42% (260mg). 1 H NMR (300MHz, CDCl 3 ): δ=7.88(d, J=5.4Hz, 1H), 7.82(t, J=8.1Hz, 1H), 7.65(s, 1H), 7.48(d, J=8.7Hz, 1H), 6.88(t ,J=6.6Hz,1H),6.15(s,1H),2.49(s,3H),2.29(s,3H). 13 C NMR (75MHz, CDCl 3 ): δ=152.0, 147.8, 142.9, 136.6, 136.0, 129.1, 122.9, 117.7, 114.4, 111.3, 14.0, 10.9. 19 F NMR (470MHz, CDCl 3 ): δ=-141.5(d, J=30.6Hz, 1F), -141.7(d, J=30.1Hz, 1F), -145.7(d, J=24.0Hz, 1F), -145.8(d, J= 24.4Hz,1F).HRMS(APCI)Calcd.For C 12 h 13 B 2 f 4 N 4 [M+H] + :311.1262, found 311.1260.
Embodiment example 3
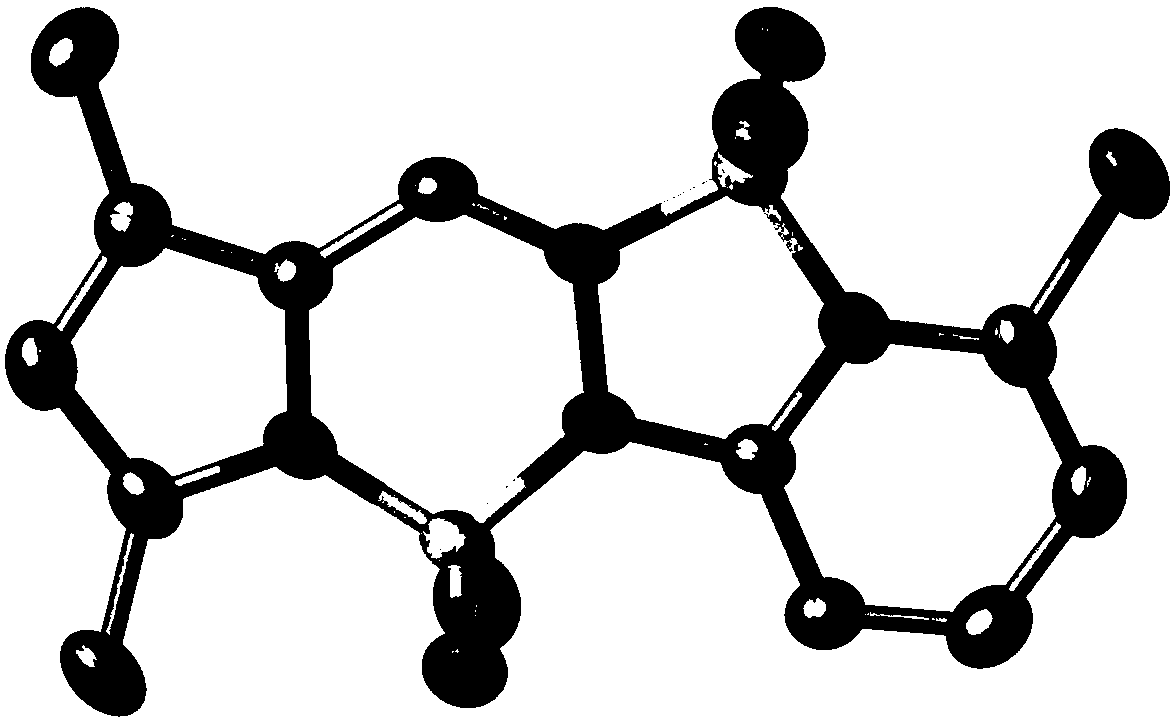
[0061] Synthesis of strong fluorescent dye 1ca from pyrrole pyridinehydrazine difluoroboron:
[0062]
[0063] Carry out according to the method for embodiment case 1, difference is to set out from 2,4-dimethyl-3-ethylpyrrole aldehyde (304mg, 2mmol) and pyridine hydrazine (230mg, 2.1mmol), the productive rate of preparation 1ca is 46 % (310 mg). 1 H NMR (300MHz, CDCl 3 ): δ=7.88(d, J=5.7Hz, 1H), 7.80(t, J=7.5Hz, 1H), 7.61(s, 1H), 7.48(d, J=8.7Hz, 1H), 6.87(t ,J=6.6Hz,1H),2.49-2.42(m,5H),2.23(s,3H),1.08(t,J=7.5Hz,3H). 13 C NMR (125MHz, CDCl 3 ): δ=151.9, 146.2, 142.7, 136.0, 133.4, 130.7, 128.5, 122.1, 114.2, 111.3, 17.2, 14.8, 12.0, 9.2. 19 F NMR (470MHz, CDCl 3 ):δ=-141.3(d,J=26.3Hz,1F),-141.5(d,J=29.1Hz,1F),-145.7(d,J=23.0Hz,1F),-145.8(d,J=23.0Hz,1F) 23.5Hz,1F).HRMS(APCI)Calcd.For C 14 h 16 B 2 f 3 N 4 [M-F] + :319.1513,found 319.1522.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com