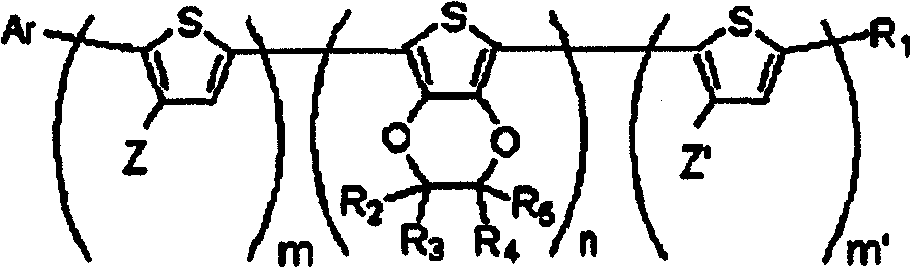
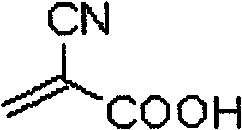
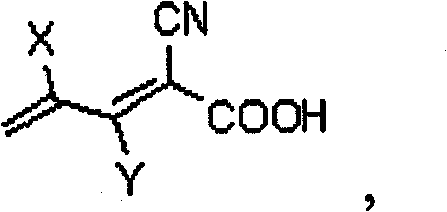
Novel organic dye and method for preparing same
A technology of organic dyes and dyes, applied in the directions of organic dyes, sulfur dyes, azo dyes, etc., can solve the problems of low conversion efficiency, low driving stability, etc., and achieve high efficiency, high molar absorption coefficient, and lower dye synthesis costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0202] Synthesis of the compound of embodiment 1 chemical formula 1a
[0203] (1-1) (2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)trimethylstannane (0.40g, 1.34mmol), chemical formula 2a After the compound (0.75g, 1.12mmol), Pd(PPh3)4 (0.065g, 0.056mmol) was dissolved in THF (40ml), it was refluxed under nitrogen atmosphere for 8 hours. Next, after extracting the organic layer with dichloromethane and water, distillation, and column chromatography (eluent-MC:Hx=1:4), the compound of chemical formula 4a was synthesized.
[0204] 1 H NMR (CDCl3): [ppm] = 0.96 (m, 6H), 1.29 (m, 4H), 1.96 (m, 4H), 2.55 (m, 8H), 2.62 (m, 4H), 4.68 (s, 2H ), 4.71(s, 2H), 6.71(m, 2H), 6.96(s, 1H), 7.08(s, 1H), 7.18(m, 4H), 7.38(m, 2H), 7.45(m, 6H) , 7.55(d, 3JHH=8.8Hz, 2H).
[0205] (1-2) After the compound of chemical formula 4a (0.87g, 1.2mmol) was dissolved in DMF (20ml), POCl3 (0.13ml 1.44mmol) was slowly added dropwise at 0°C, and stirred at 80°C for 4 hours. After the stirring was completed, t...
Embodiment 2
[0209] Synthesis of the compound of embodiment 2 chemical formula 1b
[0210] (2-1) (2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)trimethylstannane (0.40g, 1.34mmol), chemical formula 2b After the compound (1g, 1.12mmol) and Pd(PPh3)4 (0.065g, 0.056mmol) were dissolved in THF (40ml), it was refluxed under nitrogen atmosphere for 8 hours. Next, after extracting the organic layer with dichloromethane and water, distillation, and column chromatography (eluent-MC:Hx=1:4), the compound of chemical formula 4b was synthesized.
[0211] 1 H NMR (CDCl3): [ppm] = 0.96 (m, 6H), 1.29 (m, 4H), 1.44 (s, 12H), 1.96 (m, 4H), 2.55 (m, 8H), 2.62 (m, 4H ), 4.68(s, 2H), 4.71(s, 2H), 6.91(s, 1H), 6.96(s, 1H), 7.08(s, 1H), 7.18(m, 6H), 7.38(m, 6H) , 7.55(m, 4H), 7.82(d, 3JHH=8.8Hz, 2H).
[0212] (2-2) After the compound of chemical formula 4a (1.14g, 1.2mmol) was dissolved in DMF (20ml), POCl3 (0.13ml1.44mmol) was slowly added dropwise at 0°C, and stirred at 80°C for 4 hours. After the stirring wa...
Embodiment 3
[0216] Synthesis of the compound of embodiment 3 chemical formula 1e
[0217] (3-1) (2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)trimethylstannane (0.40g, 1.34mmol), 5- Bromo-2-(4-(2,2-diphenylethenyl) phenyl)-3-hexylthiophene (compound of chemical formula 2e) (0.67g, 1.34mmol), Pd(PPh3) 4 (0.065g, 0.056 mmol) was dissolved in THF (40ml), and refluxed under nitrogen atmosphere for 8 hours. Next, after extracting the organic layer with dichloromethane and water, distillation, and column chromatography (eluent-MC:Hx=1:4), the compound of chemical formula 4e was synthesized.
[0218] 1 H NMR (CDCl3): [ppm] = 0.88 (m, 3H), 1.29 (m, 2H), 1.86 (m, 2H), 2.55 (m, 4H), 2.62 (m, 2H), 4.68 (s, 2H ), 4.71(s, 2H), 6.71(s, 1H), 6.96(s, 1H), 7.08(s, 1H), 7.18(m, 4H), 7.38(m, 2H), 7.45(m, 6H) , 7.55(d, 3JHH=8.8Hz, 2H).
[0219] (3-2) After the compound of chemical formula 4e (1.14g, 1.2mmol) was dissolved in DMF (20ml), POCl3 (0.13ml 1.44mmol) was slowly added dropwise at 0°C, and stirred a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com