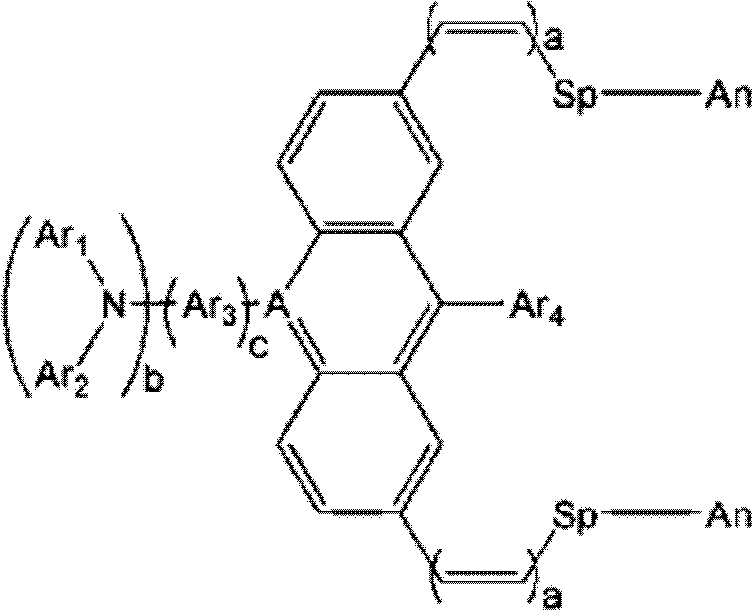
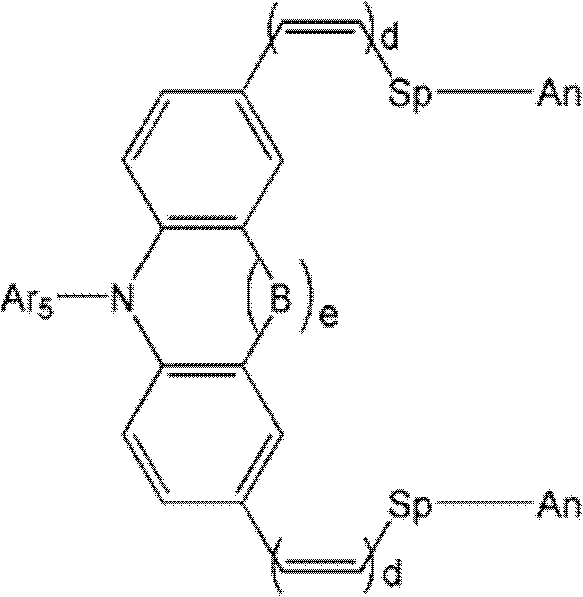
Novel Organic Dye And Preparation Method Thereof
A technology of organic dyes and dyes, applied in the directions of organic dyes, anthracene dyes, azo dyes, etc., can solve the problems of low conversion efficiency, low driving stability, etc., and achieve the effect of improving efficiency and high molar absorption coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0410] Embodiment 1: the synthesis of chemical formula 8
[0411] 1) Synthesis of intermediate (8a)
[0412] 2,7-dibromo-9-phenylacridine (2,7-dibromo-9-phenylacridine), 3',4-dihexyl-2,2'-dithiophen-5-ylboronic acid (3', 4-dihexyl-2, 2′-bithiophen-5-ylboronic acid), Pd(PPh 3 ) 4 and 2M of K 2 CO 3 The aqueous solution was mixed in dimethylformamide (DMF) and refluxed for 12 hours. The resulting reaction solution was cooled, water (30 ml) and brine were added, and the organic layer was separated and purified to obtain an intermediate (10a) having the following chemical structural formula.
[0413] [Intermediate 8a]
[0414]
[0415] 2) Synthesis of intermediate (8b)
[0416] The intermediate (8a) produced above was added to an absolute ethanol solution, and n-BuLi was further added under argon. After 3 hours, DMF was added to the resulting reaction product at 0° C. under argon gas, followed by washing with 5% KOH. The resulting reaction solution was dried over magne...
Embodiment 2
[0424] Embodiment 2: the synthesis of compound formula 10
[0425] 1) Synthesis of intermediate (10a)
[0426] Instead of 3',4-dihexyl-2,2'-dithiophen-5-ylboronic acid use 7-(5-(5,5-dimethyl-1,3-di Alkyl-2-yl)thieno[3,2-b]thiophen-2-yl)-2,3-dihydrothiophene[3,4-b][1,4]di In-5-ylboronic acid was carried out in the same manner as in Example 1 above except that, to obtain an intermediate (10a) of the following chemical structural formula.
[0427] [Intermediate 10a]
[0428]
[0429] 2) Synthesis of intermediate (10b)
[0430] In 2) of Example 1 above, the intermediate (10a) produced above was dissolved in tetrahydrofuran (THF), and trifluoroacetic acid and water were added dropwise to the resulting solution, followed by stirring for 4 hours under a nitrogen atmosphere.
[0431] After completion of the stirring, the resulting reaction solution was extracted with dichloromethane and water, and the organic layer was evaporated, and purified by column chromatography to obta...
Embodiment 3
[0439] Embodiment 3: the synthesis of chemical formula 18
[0440] 1) Synthesis of intermediate (18a)
[0441] Tetraethyl(9-phenylacridine-2,7-diyl)bis(methylene)diphosphate and 7-(5-(5,5-dimethyl-1,3-di Alkyl-2-yl)thieno[3,2-b]thiophen-2-yl)-2,3-dihydrothieno[3,4-b][1,4]di In-5-carbaldehyde was subjected to Wittig reaction in THF solvent in the presence of potassium tert-butoxide to obtain intermediate (18a) of the following chemical structural formula.
[0442] [Intermediate 18a]
[0443]
[0444] 2) Synthesis of intermediate (18b)
[0445] The intermediate (18b) having the following chemical structural formula was obtained in the same manner as in Example 1 except that the intermediate (18a) produced above was used instead of the intermediate (8a) in Example 1.
[0446] [Intermediate 18b]
[0447]
[0448] 3) Synthesis of compound formula 18
[0449] In the above-mentioned Example 1, except that the intermediate (18b) produced above was used instead of the int...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com