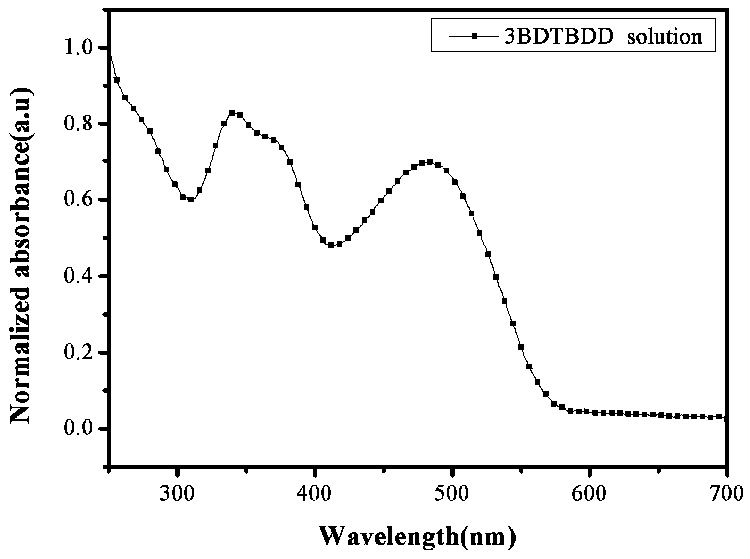
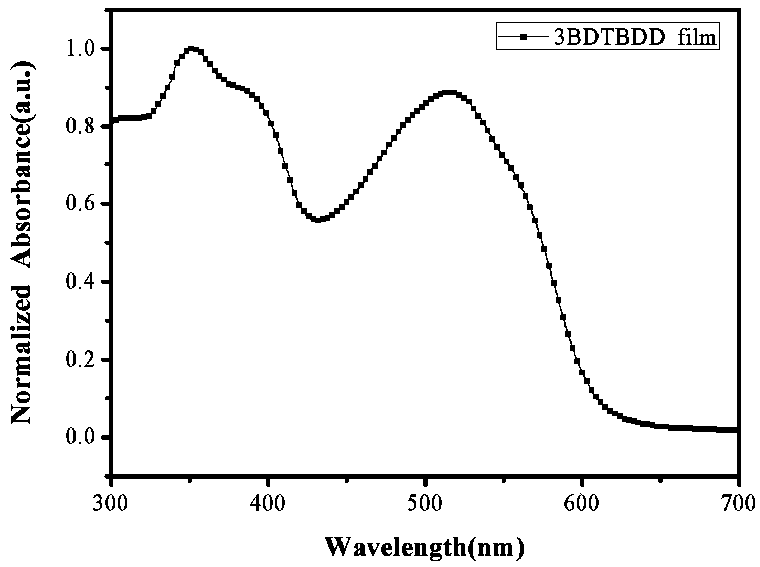
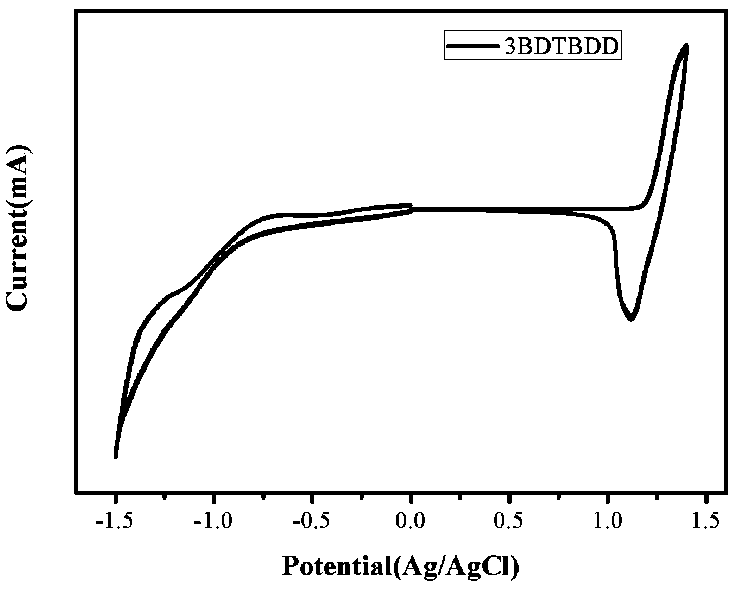
Synthesis and application of (D-A)n+1D type oligomer photovoltaic donor material based on benzodithiophene-4,8-dione
A technology of dithiophene and photovoltaic materials, which is applied in the application field of oligomer solar cell devices, can solve the problems of unclear molecular structure and performance, insufficient manifestation of advantages, and low energy conversion efficiency of devices, so as to achieve high-efficiency energy conversion Efficiency, high molar absorptivity, and the effect of improving short-circuit current
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Synthesis of 4,8-dibromo-bis(2-ethylhexyl)benzo[1,2-c:4,5-c']dithiophene-4,8-dione (M1)
[0062]
[0063] 1.1 Synthesis of 2,5-dibromo-3,4-dicarboxythiophene
[0064] Add 3,4-dicarboxythiophene (5g, 29.04mmol) and 40mL glacial acetic acid into a 100mL one-necked flask in turn, stir at room temperature, add liquid bromine (8.9mL, 174.24mol) into a 25mL constant pressure dropping funnel, Slowly drop into the reaction flask, react for 12h. After the reaction was finished, the reaction solution was poured into 500mL saturated sodium bisulfate solution and stirred until a beige solid was precipitated, filtered under reduced pressure, and dried to obtain a beige solid (8.2g, 85.6%), the chemical formula was C 6 h 2 Br 2 o 4 S, MS m / z: [M1] 329.8.
[0065] 1.2 Synthesis of 2,5-dibromo-3,4-diacylchlorothiophene
[0066] Add 2,5-dibromo-3,4-dicarboxythiophene (2g, 6.06mol) and 50mL of dry dichloromethane into a 100mL one-necked flask in turn, stir at room temperature, a...
Embodiment 2
[0070] 4,8-bis-(5-bromothienyl)-bis(2-ethylhexyl)benzo[1,2-c:4,5-c′]dithiophene-4,8-dione (M2) Synthesis
[0071]
[0072] 2.1 Synthesis of 4,8-dithienyl-bis(2-ethylhexyl)benzo[1,2-c:4,5-c′]dithiophene-4,8-dione
[0073] Add M1 (0.5g, 0.83mmol), tributylstannylthiophene (0.93g, 2.5mmol), tetrakis(triphenylphosphine)palladium (96mg), and 30mL toluene into a 100mL two-necked flask in turn, and nitrogen deoxidation . The temperature was raised and transferred to 110° C. and the reaction was stirred for 12 h. The reaction was stopped, and after the reactant was cooled to room temperature, it was poured into 100 mL of distilled water and extracted with dichloromethane (3 x 30 mL). The organic phases were combined, dried with anhydrous magnesium sulfate, filtered, and the filtrate was distilled under reduced pressure to remove the solvent, and the residue was subjected to column chromatography with petroleum:dichloromethane mixed solution (v / v, 5:1) as eluent After separation...
Embodiment 3
[0077] Synthesis of 2-trimethyltin-4,8-bis(5-(2-ethylhexyl)thienyl)benzo[1,2-b:4,5-b']dithiophene (M3)
[0078]
[0079] 4,8-bis(5-(2-ethylhexyl)thienyl)benzo[1,2-b:4,5-b']dithiophene (0.3g, 0.52mmol), 15mL anhydrous THF Add it into a 50mL two-necked bottle, pass nitrogen to deoxygenate, transfer to -78°C and cool for 15min, slowly inject n-BuLi (0.23mL, 0.57mmol, 2.5M / L THF solution), continue to react at -78°C for 2h, inject Trimethyltin chloride (0.57mL, 0.57mmol, 1M / L THF solution), continued to react at -78°C for 0.5h, then transferred to room temperature for 12h. The reaction was stopped, quenched by adding 10 mL of water, the reaction solution was poured into 100 mL of distilled water, and extracted with dichloromethane (3x 30 mL). The combined organic phases were dried with anhydrous magnesium sulfate, filtered, and the filtrate was distilled under reduced pressure to remove the solvent and dried without further treatment to obtain 3.7 g of light yellow viscous liq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface roughness | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com