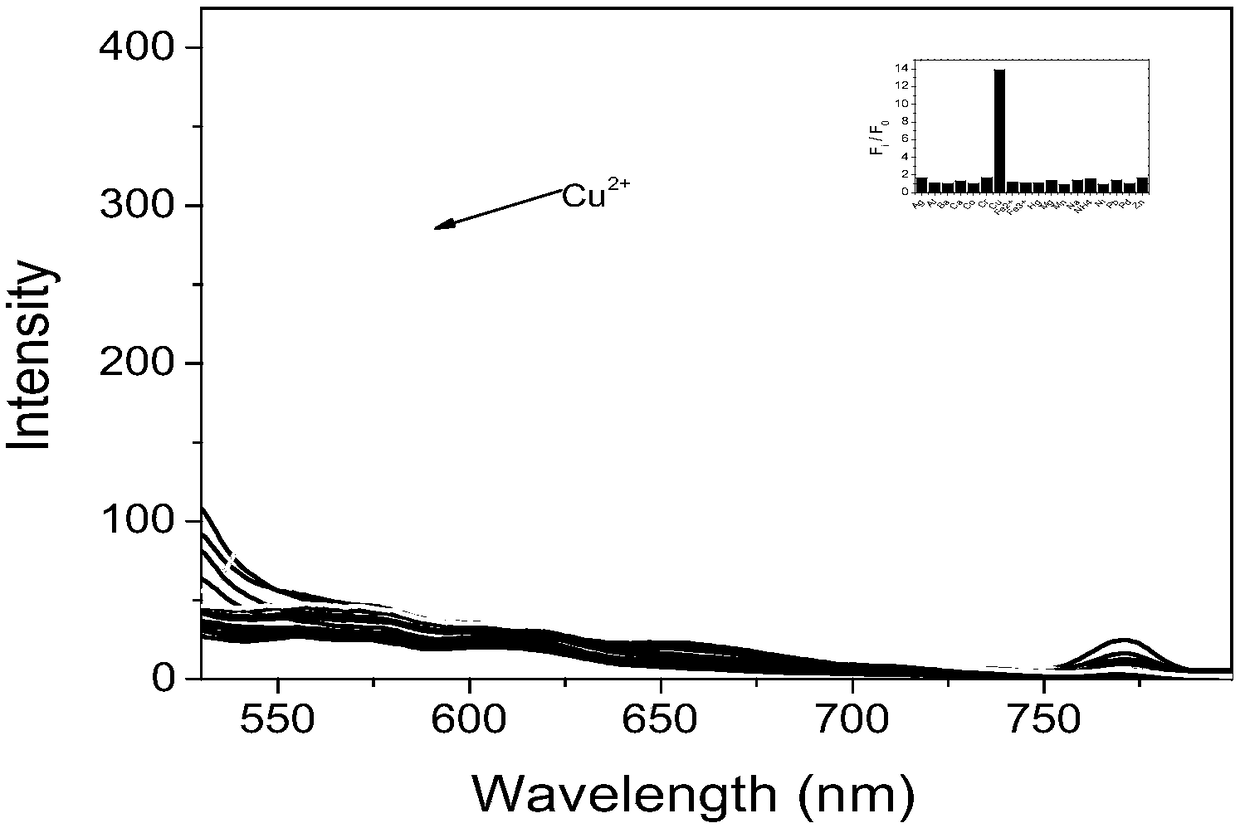
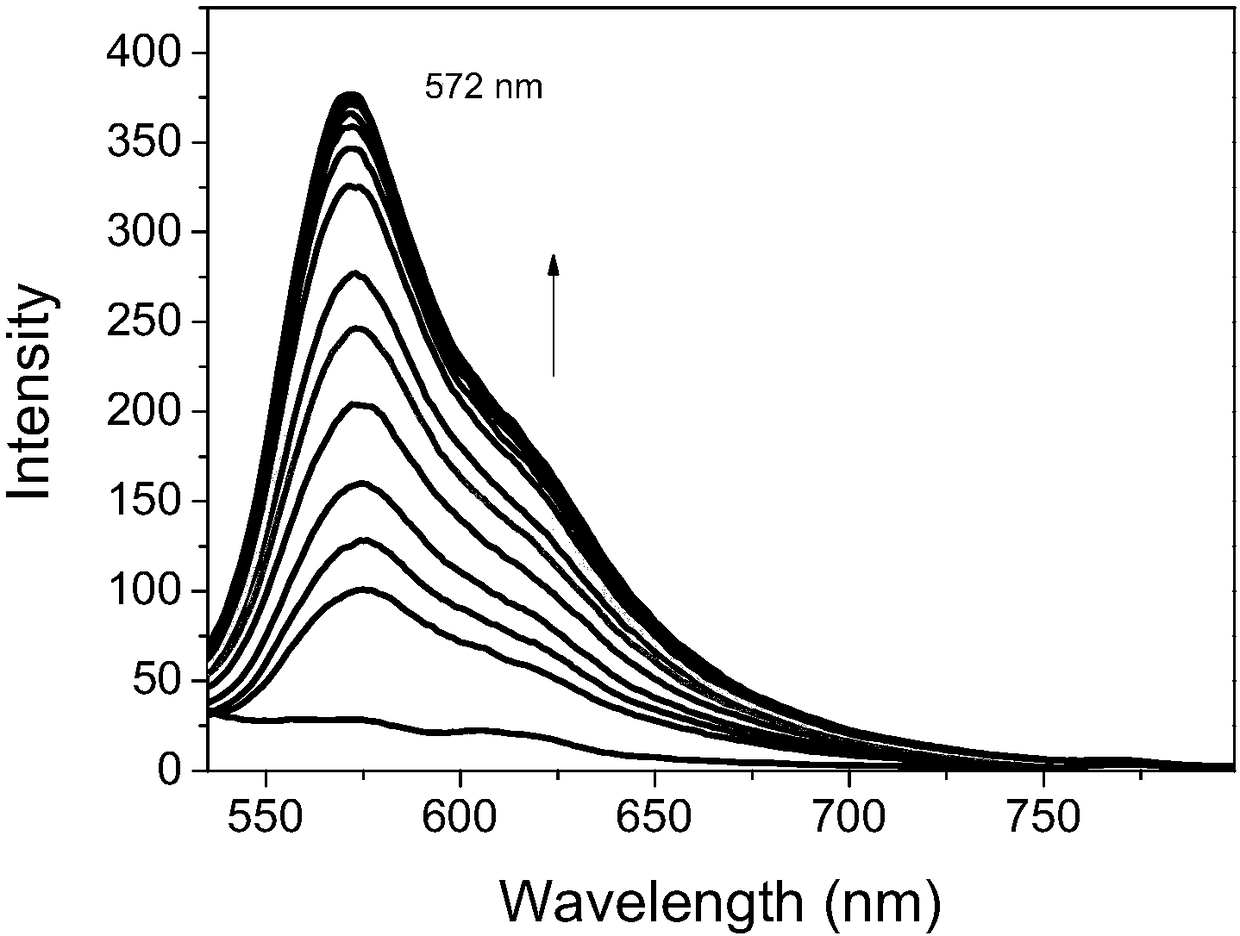
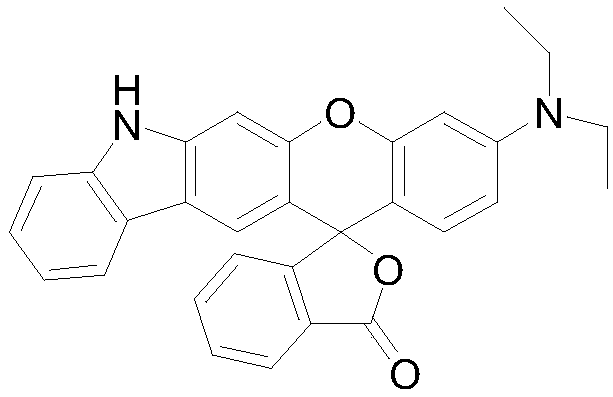
Preparation method and application of fluorescent dye of carbazole-rhodamine hybrid structures
A fluorescent dye and carbazole technology, which is applied in the field of preparation of fluorescent dyes based on carbazole-rhodamine hybrid structure, can solve the problems of non-existence, prone to photobleaching, and large interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, the preparation of carbazole-rhodamine hybrid fluorescent dye 1
[0024] The preparation steps are as follows:
[0025] 1) Add 2-hydroxycarbazole (1.83g, 10mmol) and 2-(4-diethylamino-2-hydroxybenzoyl) benzoic acid (3.14g, 10mmol) into 60mL of methanesulfonic acid and stir in 60°C oil The reaction was heated in a bath for 5 hours, and then heated in an oil bath at 80° C. for 10 hours to obtain a product solution.
[0026] 2) Add 600mL water to the reacted product solution, add appropriate amount of NaHCO 3 The solid neutralized the reaction solution, extracted with dichloro to collect the organic phase, and washed with anhydrous Na 2 SO 4 After drying and filtering, the obtained solid crude product was passed through a silica gel column with dichloromethane and ethyl acetate to obtain carbazole-rhodamine hybrid fluorescent dye 1, 3.31 g of powder white solid powder, yield 72%, melting point: 238-242 ℃.
[0027] 1 H NMR (400MHz, CDCl 3 )δ=8.23(d,J=6....
Embodiment 2
[0028] Embodiment 2, the preparation of carbazole-rhodamine hybrid fluorescent dye 1
[0029] 1) Add 2-hydroxycarbazole (1.83g, 10mmol) and 2-(4-diethylamino-2-hydroxybenzoyl) benzoic acid (3.46g, 11mmol) into 50mL methanesulfonic acid and stir, 65°C oil The reaction was heated in a bath for 5 h, and then heated in an oil bath at 85° C. for 5 h.
[0030] 2) Add 600mL water to the reaction solution, add appropriate amount of NaHCO 3 The solid neutralized the reaction solution, extracted with dichloro to collect the organic phase, and washed with anhydrous Na 2 SO 4After drying and filtering, the obtained solid crude product was passed through a silica gel chromatography column with dichloromethane and ethyl acetate to obtain carbazole-rhodamine hybrid fluorescent dye 1, 3.12 g of off-white solid powder, and the yield was 67.8%. Melting point: 238-242°C. Progen spectrum and carbon spectrum are the same as embodiment 1.
Embodiment 3
[0031] Embodiment 3, the preparation of carbazole-rhodamine hybrid fluorescent dye 1
[0032] 1) Add 2-hydroxycarbazole (1.83g, 10mmol) and 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid (3.76g, 12mmol) into 50mL of methanesulfonic acid and stir in 80°C oil The reaction was heated in a bath for 10 h, and then heated in an oil bath at 95° C. for 5 h.
[0033] 2) Add 600mL water to the reaction solution, add appropriate amount of NaHCO 3 The solid neutralized the reaction solution, extracted with dichloro to collect the organic phase, and washed with anhydrous Na 2 SO 4 After drying and filtering, the obtained solid crude product was passed through a silica gel chromatography column with dichloromethane and ethyl acetate to obtain carbazole-rhodamine hybrid fluorescent dye 1, 3.22 g of powdery white solid powder, and the yield was 70.0%. Melting point: 238-242°C. Progen spectrum and carbon spectrum are the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com