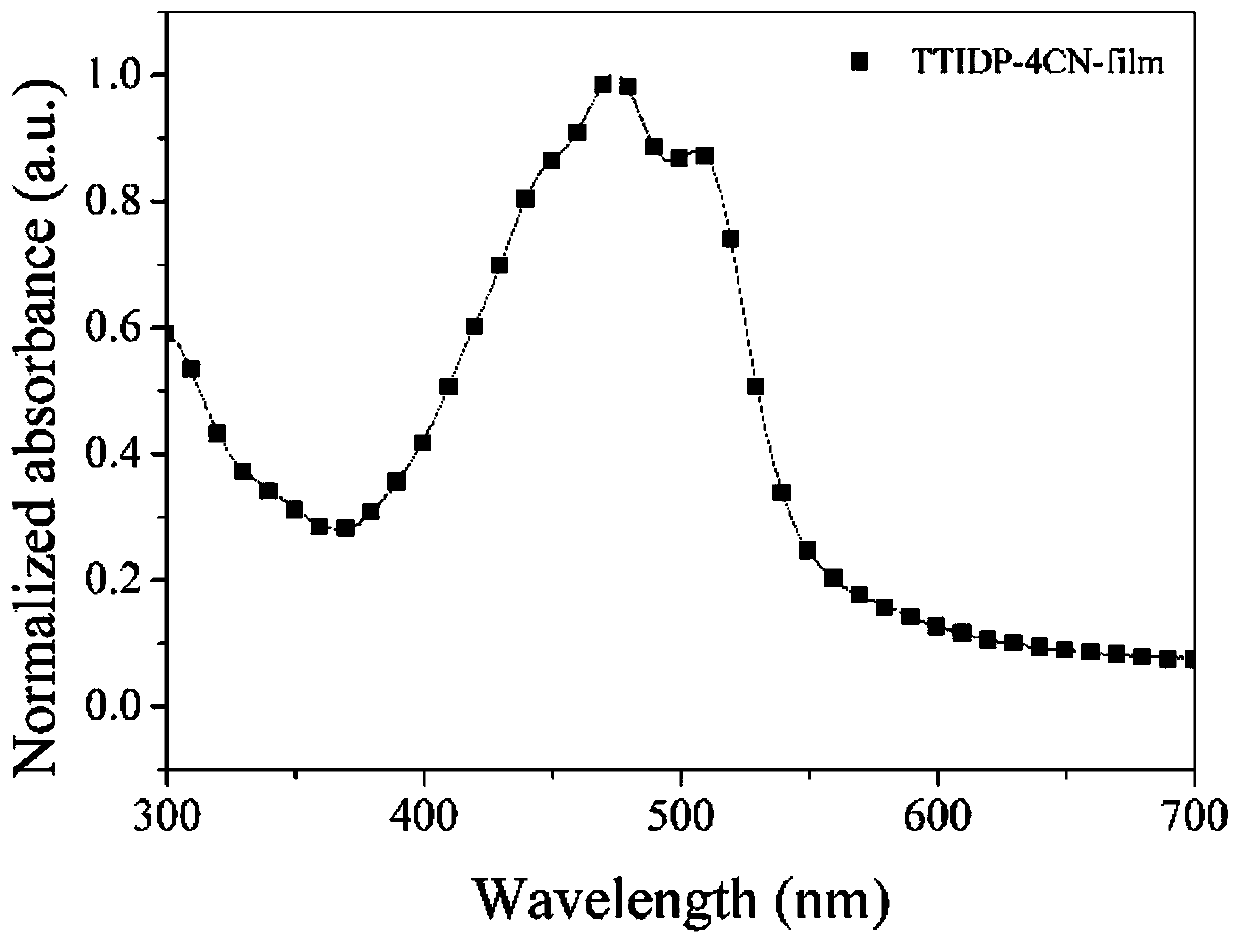
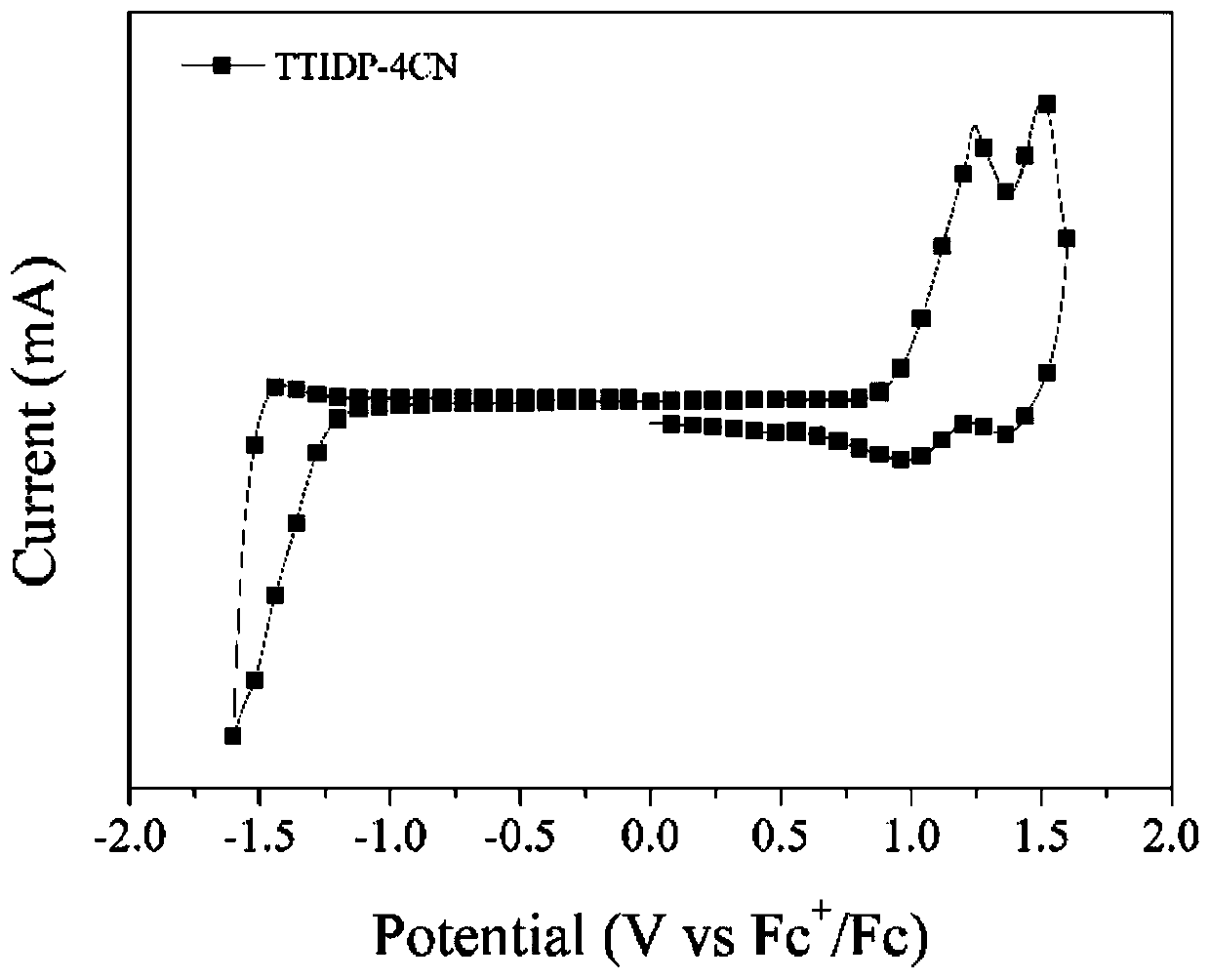
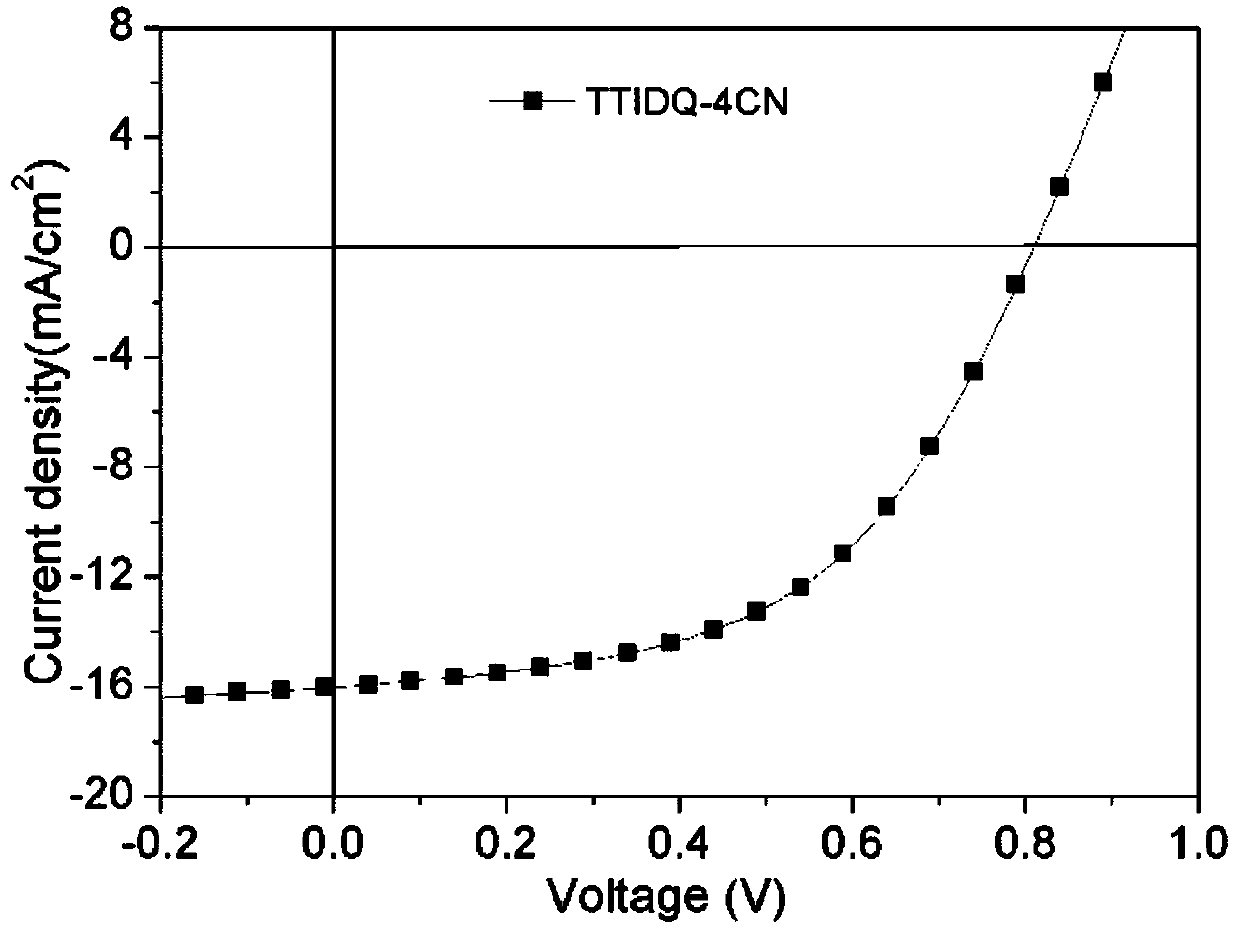
D(pi-A)2 type small molecular donor material based on pyrazine indole terminal receptors, and preparation method and application thereof
A technology of small molecule donor, pyrazine indole, which is applied in semiconductor/solid-state device manufacturing, electrical solid-state devices, semiconductor devices, etc., can solve problems such as low carrier mobility, narrow absorption spectrum, and poor stability, and achieve Effects of high mobility, broad absorption, and enhanced absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] D(π-A) 2 Synthesis of small molecule donor material intermediates and target molecules (M1, M2, M3, M4, M5, IDP-2CN, IDQ-2F, IDQ-2Cl, TTIDP-2CN, TTIDP-2F and TTIDP-2Cl) Synthetic, its synthetic route is as follows:
[0064]
[0065] 1.1 Synthesis of 3,6-bis(octylthio)-thieno[3,2-b]thiophene (M1):
[0066]
[0067] Add 3,6-dibromothiophene[3,2-b]thiophene (5.0g, 16.9mmol) in a 200mL single-necked bottle, n-C 8 h 17 SH(1-octylthiol) (5.4g, 36.9mmol), DIEA (4.8g, 36.9mmol), DPPF (370mg, 0.7mmol), Pd 2 (dba) 3 (310mg, 0.3mmol), add 80mL toluene, vacuumize three times, under the protection of nitrogen, reflux and stir at 110°C for 24h, after the reaction is completed, the reactant is cooled to room temperature, the mixture is poured into water, extracted with dichloromethane (3 ×50mL), washed three times with water, dried with anhydrous magnesium sulfate, filtered, and the organic solvent was removed under reduced pressure, and the product was separated by column ...
Embodiment 2
[0089] Example 2 Synthesis of Small Molecule Donor TTIDP-4CN
[0090]
[0091] In a 100mL two-necked flask, sequentially add compound M4 (0.2g, 0.218mmol), compound IDP-4CN (0.189g, 0.479mmol), three (dibenzylideneacetone) dipalladium (5.98mg), three (o-tolyl ) phosphorus (7.94mg), toluene 10mL, under the condition of nitrogen protection, stirred and refluxed in an oil bath at 110°C for 24h. Stop the reaction, cool to room temperature, pour the reaction solution into 40mL water, extract with dichloromethane (3×25mL), dry over anhydrous magnesium sulfate, filter, spin off the organic solvent under reduced pressure, and pass the crude product through absolute ethanol / chloroform Recrystallization gave a red solid (282 mg, yield 84.9%). 1 H NMR (400MHz, CDCl 3 )δ8.46(d, J=2.2Hz, 1H), 7.97(dd, J=8.8, 2.1Hz, 1H), 7.44(d, J=9.0Hz, 1H), 7.32(s, 2H), 4.32( s,2H),2.89(s,2H),1.87(s,2H),1.66-1.14(m,30H),0.86(d, J=35.9Hz,7H).
Embodiment 3
[0092] Example 3 Synthesis of Small Molecule Donor TTIDQ-4F
[0093]
[0094] In a 100mL two-necked flask, sequentially add compound M4 (0.2g, 0.218mmol), compound IDQ-2F (0.214g, 0.479mmol), tris(dibenzylideneacetone) dipalladium (5.98mg), tris(o-tolyl ) Phosphorus (7.94mg), toluene 10mL, under the condition of nitrogen protection, stirred and refluxed in an oil bath at 110°C for 24h. Stop the reaction, cool to room temperature, pour the reaction solution into 40mL water, extract with dichloromethane (3×25 mL), dry over anhydrous magnesium sulfate, filter, spin off the organic solvent under reduced pressure, and pass the crude product through anhydrous ethanol / trichloro Recrystallization from methane gave a red solid (225 mg, 78.1% yield). 1 H NMR (400MHz, CDCl 3 ) δ8.68(s,1H),8.07-7.97(m,2H),7.85(d,J=19.4Hz,1H),7.51-7.45(m,2H),7.36 (d,J=3.9Hz,1H ),4.46(s,2H),2.98(t,J=7.3Hz,2H),1.95(s,2H),1.66(s,2H), 1.40(s,7H),1.25(s,21H),0.84 (d,J=20.9Hz,8H),0.07(s,14H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com