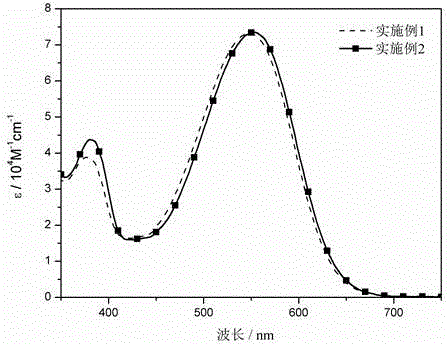
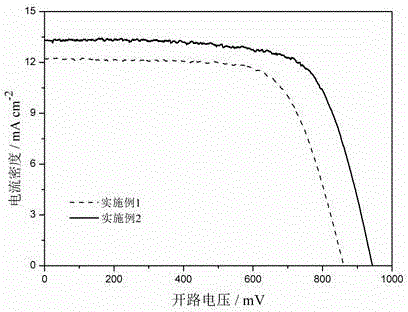
Dithiophene pyrrole bridge-indoline organic dyes as well as preparation method and application thereof
A technology of dithiophene pyrrole bridges and indolines, applied in the fields of organic dyes, organic chemistry, azo dyes, etc., can solve the problems of unfavorable large-scale commercial application of DSSC, low molar absorptivity, and high preparation cost, and achieve Improve the photoelectric conversion efficiency, improve the molar absorptivity, and improve the effect of absorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method of the dithiophenepyrrole bridge-indoline organic dyes, using dithiophenepyrrole bromaldehyde substituted with indoline boroester, tetrakistriphenylphosphine, potassium carbonate and alkoxytriphenylamine as The raw materials are sequentially subjected to Suzuki coupling reaction and Knoevenagel condensation reaction to obtain the target product. The steps are as follows:
[0030] 1) Synthesis of fluorenyl indoline-dithiophene pyrrole carboxaldehyde
[0031] Under the condition of nitrogen protection, in a 100 mL two-necked bottle, add 146 mg (0.2 mmol) of hexyloxytriphenylamine-substituted dithiophene pyrrole bromaldehyde, 267 mg (0.5 mmol) of fluorenyl indoline borolipid, 23.1 mg (0.02 mmol) of tetraphenyltriphenylphosphine, 276 mg (2 mmol) of potassium carbonate solution with a concentration of 2M and 30 mL of ethylene glycol dimethyl ether. After cooling the obtained reaction solution a to room temperature, add 15 mL of water to quench , and the...
Embodiment 2
[0044] A preparation method of the dithiophenepyrrole bridge-indoline organic dyes, using dithiophenepyrrole bromaldehyde substituted with indoline boroester, tetrakistriphenylphosphine, potassium carbonate and alkoxytriphenylamine as The raw materials are sequentially subjected to Suzuki coupling reaction and Knoevenagel condensation reaction to obtain the target product. The steps are as follows:
[0045] 1) Synthesis of tripolyindenyl indoline-dithiophene pyrrole formaldehyde
[0046] Under the condition of nitrogen protection, in a 100 mL two-necked bottle, add 146 mg (0.2 mmol) of hexyloxytriphenylamine-substituted dithiophene pyrrole bromide, 438 mg (0.5 mmol) ), 23.1 mg (0.02 mmol) of tetraphenyltriphenylphosphine, 276 mg (2 mmol) of potassium carbonate solution with a concentration of 2M and 30 mL of ethylene glycol dimethyl ether. After cooling the obtained reaction solution a to room temperature, add 15 mL of water Quenched, then extracted with ethyl acetate, separa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com