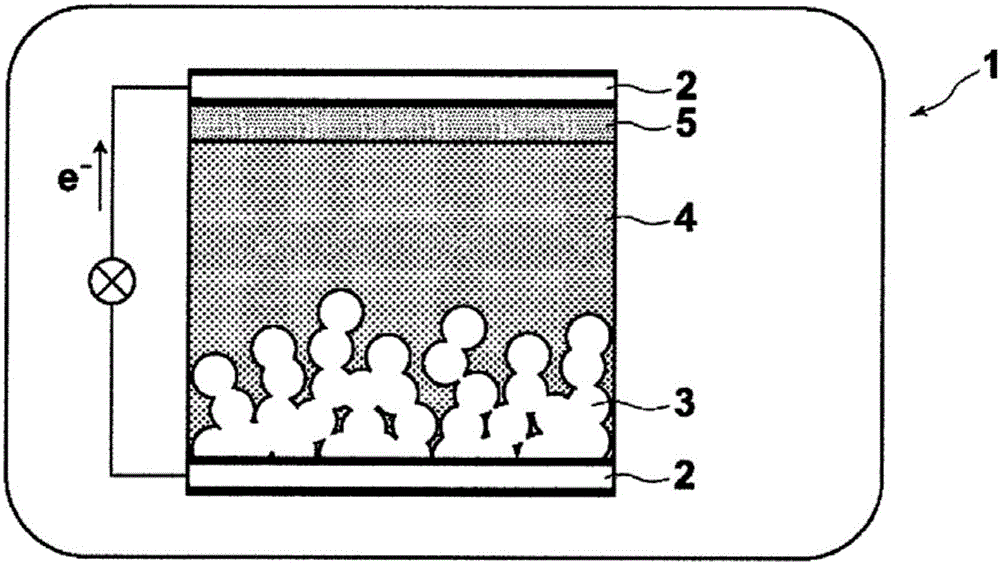
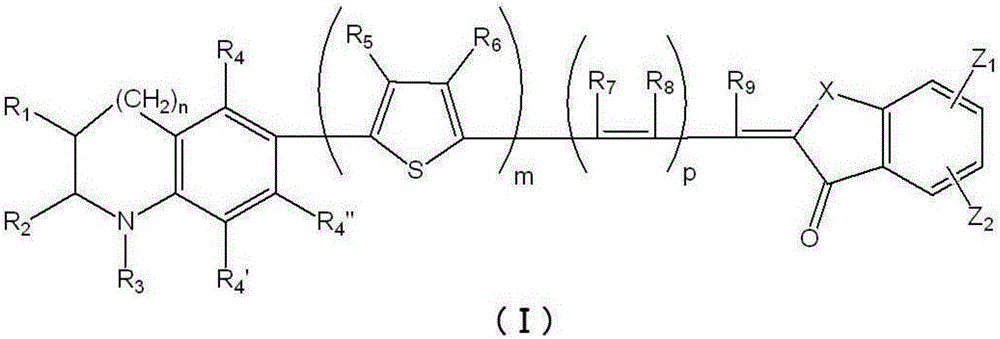
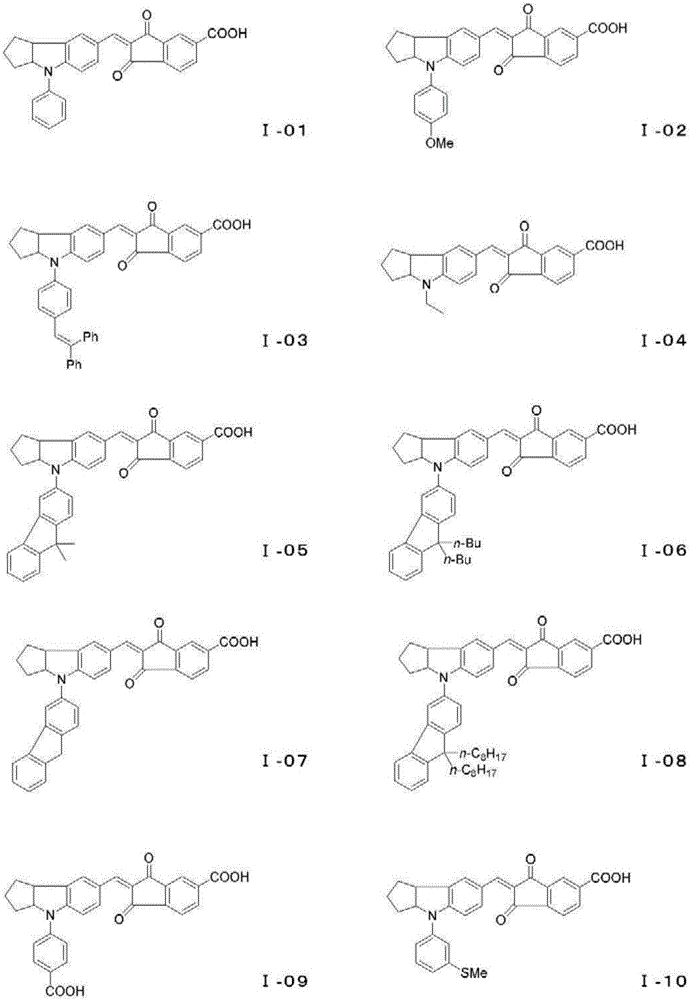
Photosensitizer and photoelectric conversion element
A technology for photoelectric conversion elements and photosensitizers, applied in the fields of photosensitizers and photoelectric conversion elements, can solve problems such as conversion efficiency and durability that have not yet reached a practical level, and achieve improved photoelectric conversion efficiency, durability, and high molar absorption coefficient. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163]
[0164] Aldehyde (B-01) (0.88 g) and intermediate (C-01) (0.50 g) were dissolved in acetic acid (10 mL), and heated and stirred at 100° C. for 4 hours. The reaction mixture was cooled to room temperature and solidified. The crude crystals were separated by filtration, and were separated by column chromatography (silica gel, developer: CHCl 3 / MeOH=100 / 1) was separated and purified, whereby 0.85 g of a pigment (I-03) was obtained as a brown solid (69% yield). λmax=545nm (CHCl 3 ).
[0165] The structure of the obtained pigment (I-03) was identified by NMR analysis.
[0166] 1 H NMR (400MHz, DMSO-d 6 )
[0167] δ (ppm) = 13.65 (1H, br.s), 8.67 (1H, br.s), 8.33 (1H, dd, J = 8.0, 7.6Hz), 8.24 (1H, d, J = 11.6Hz), 8.20 (1H,br.s),7.94(1H,dd,J=7.6,7.6Hz),7.65(1H,s),7.41-7.49(3H,m),7.30-7.38(5H,m),7.19-7.23 (4H,m),7.11(1H,s),7.07(2H,d,J=8.4Hz),6.87(1H,d,J=8.8Hz),5.07-5.11(1H,m),3.82-3.87( 1H,m),2.03-2.12(1H,m),1.69-1.83(2H,m),1.59-1.67(2H,m),1.27-1.38(1H,m)
Embodiment 2
[0168]
[0169] Using the same method as in Example 1, and using an aldehyde intermediate (B-02), a pigment (I-05) was obtained as a black solid. λmax=541nm (CHCl 3 ).
[0170] The structure of the obtained pigment (I-05) was identified by NMR analysis.
[0171] 1 H NMR (400MHz, DMSO-d 6 )
[0172] δ(ppm)=13.63(1H,br.s),8.72(1H,br.s),8.35(1H,d,J=8.0Hz),8.28(1H,br.s),8.25(1H,d, J=11.6Hz),7.95(1H,dd,J=8.0,7.6Hz),7.90(1H,d,J=8.4Hz),7.83(1H,d,J=7.2Hz),7.69(1H,s) ,7.67(1H,d,J=1.6Hz),7.56(1H,d,J=7.2Hz),7.41(1H,dd,J=8.0,1.2Hz),7.36(1H,dd,J=8.0,7.2 Hz), 7.32(1H,dd,J=8.4,7.2Hz),6.87(1H,d,J=8.4Hz),5.24-5.27(1H,m),3.90-3.94(1H,m),2.08-2.18 (1H,m),1.74-1.88(3H,m),1.64-1.73(1H,m),1.50(3H,s),1.47(3H,s),1.37-1.53(1H,m)
Embodiment 3
[0173]
[0174] Using the same method as in Example 1, and using an aldehyde intermediate (B-03), a pigment (I-06) was obtained as a black solid. λmax=546nm (CHCl 3 ).
[0175] The structure of the obtained pigment (I-06) was identified by NMR analysis.
[0176] 1 H NMR (400MHz, DMSO-d 6 )
[0177] δ (ppm) = 13.07 (1H, br.s), 8.17 (1H, br.s), 7.81 (1H, dd, J = 7.6, 7.2Hz), 7.73 (2H, d, J = 9.6Hz), 7.41 (1H,d,J=8.0Hz),7.34(1H,d,J=8.4Hz),7.27(1H,d,J=7.2Hz),7.14(1H,s),6.99(1H,s),6.92 (1H,d,J=6.8Hz),6.88(1H,d,J=8.0Hz),6.83(1H,dd,J=7.2,6.0Hz),6.80(1H,dd,J=7.2,6.4Hz) ,6.30(1H,d,J=8.4Hz),4.72-4.75(1H,m),3.36-3.40(1H,m),1.57-1.65(1H,m),1.43-1.56(4H,m),1.28 -1.35(1H,m),1.11-1.26(3H,m),0.84-0.95(1H,m),0.45-0.59(4H,m),0.16(3H,t,J=7.2Hz),0.10(3H ,t,J=7.2Hz),0.02-0.04(4H,m)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com