Electrochromic material and electrochromic device based on dynamic metal-ligand complexation
A technology of electrochromic materials and metals, applied in the direction of color-changing fluorescent materials, instruments, copper organic compounds, etc., can solve the problems of difficult preparation of electrochromic materials, low contrast, high coloring efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
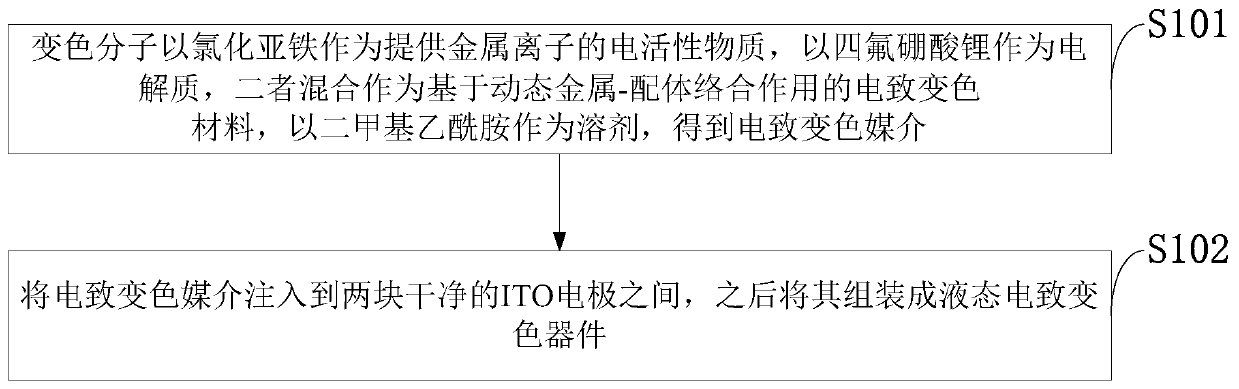
[0052] Such as figure 1 As shown, the preparation method of the electrochromic device provided by the embodiment of the present invention includes the following steps:
[0053] S101: The color-changing molecule uses ferrous chloride as the electroactive substance that provides metal ions, and lithium tetrafluoroborate as the electrolyte. The two are mixed as an electrochromic material based on dynamic metal-ligand complexation, and dimethyl ethyl amides as solvents to obtain electrochromic media;
[0054] S102: Inject the electrochromic medium between two clean ITO electrodes, and then assemble it into a liquid electrochromic device.
[0055] In a preferred embodiment of the present invention, the structure of the electrochromic device is a first electrode, an electrochromic medium, an ion exchange membrane, an auxiliary medium, and a second electrode. The structure of the liquid electrochromic device is a first electrode, an electrochromic medium and a second electrode.
...
Embodiment 1
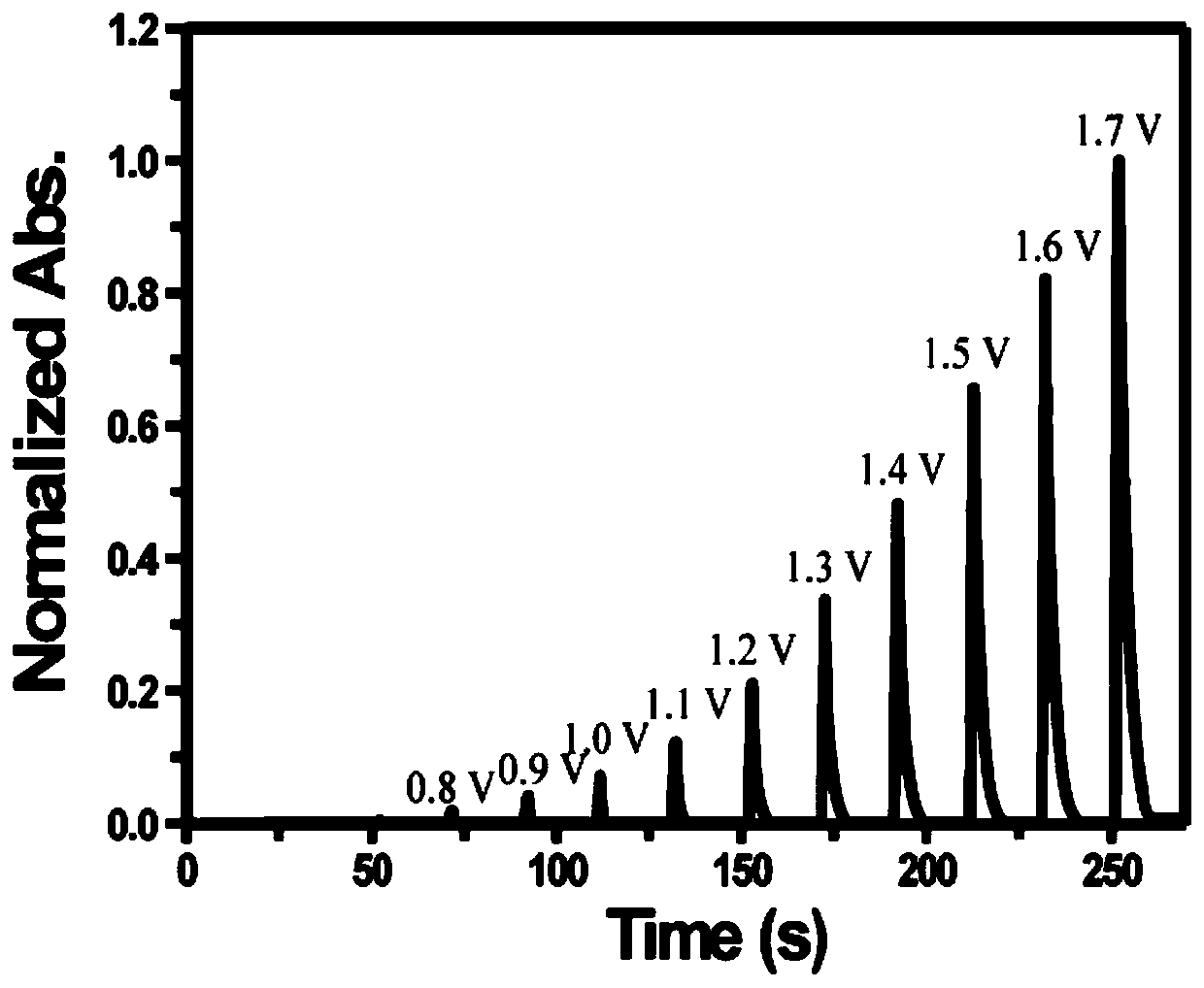
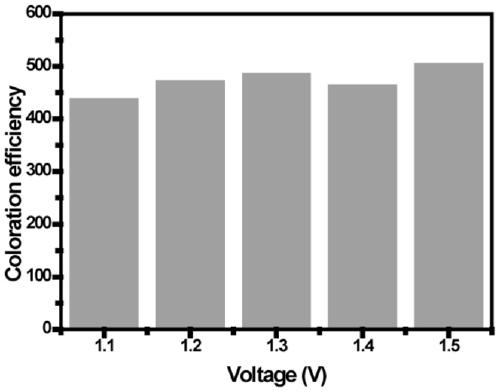
[0068]In the embodiment of the present invention, formula I is used as the color-changing molecule, cuprous iodide is used as the electroactive substance providing metal ions, and 1-butyl-3-methylimidazolium hexafluorophosphate is used as the electrolyte, and the two are mixed as the Electrochromic materials for dynamic metal-ligand complexation using acetonitrile as solvent. Wherein formula I-1 is R in general formula I 1 is N, N-diethyl, R 3 is methyl, R 4 is aminophenyl, R 2 , R 5 , R 6、 R 7 is hydrogen, Y is oxygen, and its structural formula is shown in formula I-1. Electrochromic medium preparation: 0.01mol of 1-butyl-3-methylimidazolium hexafluorophosphate as electrolyte, 106mg I-1, 10mg iodine Cuprous chloride, 64 mg of p-benzoquinone was adjusted to 10 mL with acetonitrile.
[0069]
[0070] Preparation of electrochromic device: inject the above electrochromic medium between two clean ITO electrodes, and then assemble it into a liquid electrochromic device. ...
Embodiment 2
[0072] In the embodiment of the present invention, formula II is used as the color-changing molecule, ferrous chloride is used as the electroactive material for providing metal ions, lithium tetrafluoroborate is used as the electrolyte, and the two are mixed as an electroactive material based on dynamic metal-ligand complexation. Chromogenic material with dimethylacetamide as solvent. Wherein formula Ⅱ-1 is R in formula Ⅱ 1 , R 3 is N, N-diethyl, R 2 , R 4 is hydroxyl, R 5 , R 6 , R 7 It is hydrogen, and its structural formula is shown in II-1 below. Preparation of electrochromic medium: Weigh 0.30g of lithium tetrafluoroborate, 0.2g of II-1 molecules, and dilute to 10mL with dimethylacetamide.
[0073]
[0074] Preparation of electrochromic devices: spin-coat the above-mentioned electrochromic medium onto a clean ITO-1 electrode, prepare an ion-exchange membrane with PMMA / IL (60% / 40%, v / v), and p-benzoquinone ( 3.2mg / mL) / p-phenol (3.2mg / mL) / PMMA / IL (60% / 40%, v / v) wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com