Near-infrared truxene-based conjugate dual-BODIPY fluorescent dye and preparation method thereof
A technology of trisindenyl and fluorescent dyes, which is applied in the direction of azo dyes, organic dyes, and luminescent materials, and can solve the problems of many synthesis steps, poor solubility, and limited applications, and achieve good solubility and simple and easy preparation methods , the effect of high molar absorptivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
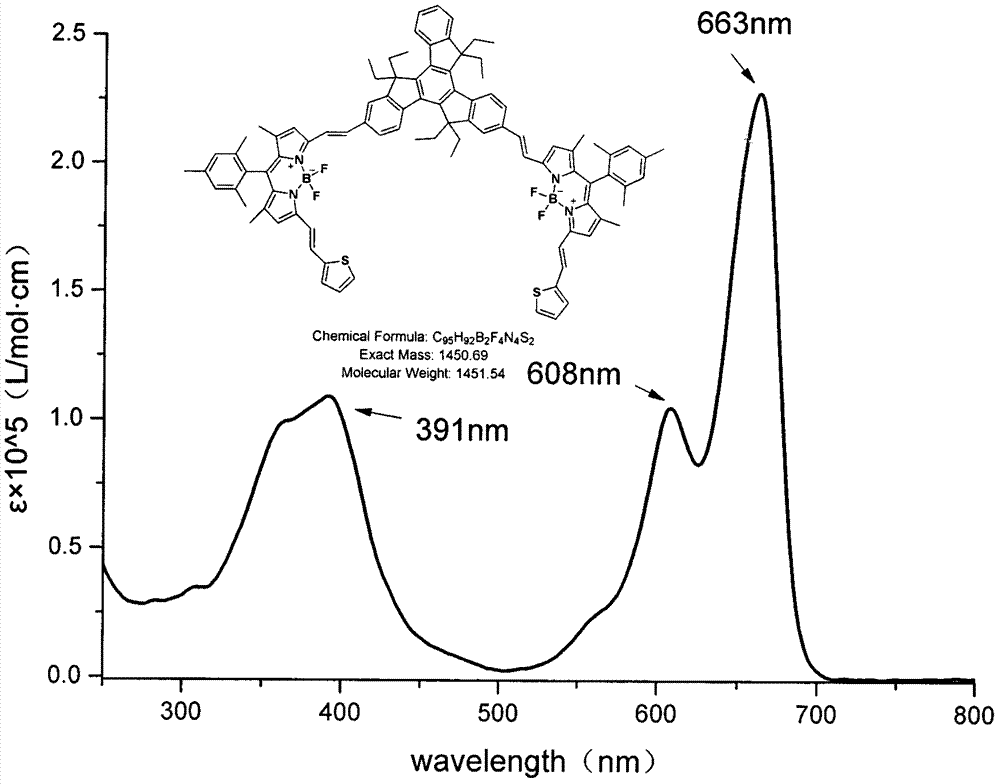
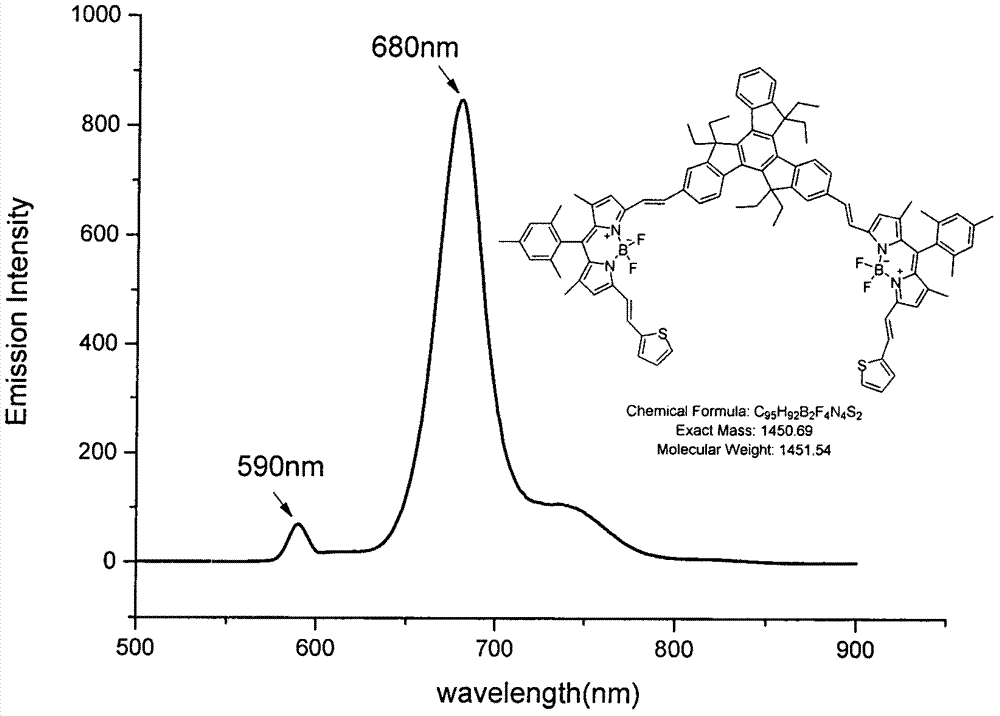
[0029] Equipped with a water trap in the round bottom flask, 1,5,7-tetramethyl-3-vinylthiophene-8-(2,4,6-trimethylbenzene)-BODIPY (120mg, 0.26mmol), 2, 6-dialdehyde-based triacinene (80mg, 0.13mmol) and p-toluenesulfonic acid (50mg) were dissolved in 25mL toluene and 2mL piperidine, the mixture was heated to reflux, and TLC was tracked to detect that the reaction of the raw materials was complete. The solvent was collected until it was evaporated to dryness, washed with water, Extract with dichloromethane, combine the organic layers, remove the organic solvent under reduced pressure, and the residue is separated and purified by silica gel column chromatography, and the eluent is (petroleum ether / CH 2 Cl 2 =1:1), to obtain compound a (17.4 mg, 28.9%) as a black-green solid. 1 H NMR: (600MHz, CDCl 3 )δ8.50-8.39(m, 3H), 7.86(d, J=24.30Hz, 2H), 7.79(d, J=12.24Hz, 2H), 7.62-7.58(m, 4H), 7.50-7.48(d , 1H), 7.44-7.34(m, 8H), 7.27(d, J=5.76Hz, 2H), 7.07(t, J=9.60Hz, 2H), 6.98(s, 4H...
Embodiment 2
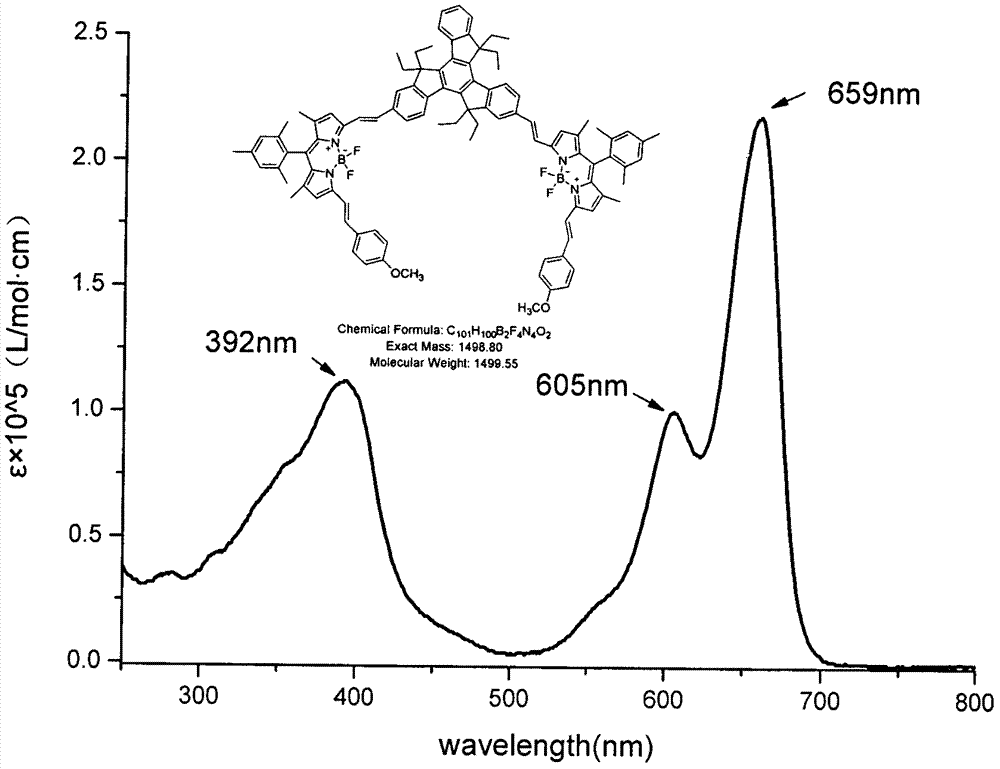
[0031] Equipped with a water trap in the round bottom flask, 1,5,7-tetramethyl-3-vinylbenzene (4-methoxy)-8-(2,4,6-trimethylbenzene)-BODIPY (150mg , 0.31mmol), 2,6-dialdehyde-based tripolyindene (92mg, 0.15mmol) and p-toluenesulfonic acid (50mg) were dissolved in 25mL toluene and 2mL piperidine, the mixture was heated to reflux, TLC tracking detection raw material reaction was complete, collected The solvent was evaporated to dryness, washed with water, extracted with dichloromethane, the organic layers were combined, and the organic solvent was removed under reduced pressure. The residue was separated and purified by silica gel column chromatography, and the eluent was (petroleum ether / CH 2 Cl 2 =1:1) to obtain compound b (22.8 mg, 15.2%) as a black-purple solid. 1 HNMR: (600MHz, CDCl 3 ( d, 6H), 7.50(d, J=6.6Hz, 1H), 7.45-7.39(m, 4H), 7.26(d, J=15.6Hz, 2H), 6.99-6.95(m, 8H), 6.70(s , 2H), 6.64(s, 2H), 3.83(s, 6H), 3.11-3.01(m, 6H), 2.37(s, 6H), 2.31-2.19(m, 6H), 2.16(s, ...
Embodiment 3
[0033] A water separator is equipped in the round bottom flask, and 1,5,7-tetramethyl-3-vinylbenzene (4-(1-piperidine))-8-(2,4,6-trimethylbenzene)- BODIPY (155mg, 0.28mmol), 2,6-dialdehyde tripolyindene (83mg, 0.14mmol) and p-toluenesulfonic acid (50mg) were dissolved in 25mL toluene and 2mL piperidine, the mixture was heated to reflux, and the reaction of raw materials was tracked and detected by TLC Completely, collect the solvent until it is evaporated to dryness, wash with water, extract with dichloromethane, combine the organic layers, remove the organic solvent under reduced pressure, and the residue is separated and purified by silica gel column chromatography, and the eluent is (petroleum ether / CH 2 Cl 2 =1:1), a black solid compound c (45.0 mg, 28.0%) was obtained. 1 H NMR: (600MHz, CDCl 3)δ8.47-8.41(m, 3H), 7.80(d, J=18.00Hz, 2H), 7.81-7.77(m, 2H), 7.66(t, J=24.00Hz, 4H), 7.59-7.57(m , 4H), 7.50(d, J=6.00Hz, 1H), 7.46-7.39(m, 4H), 7.27(d, J=18.00Hz, 2H), 6.99(s, 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com