Triphenylamine-thiophene organic dyestuff as well as preparation method and application thereof
An organic dye, triphenylamine technology, applied in the direction of organic dyes, organic chemistry, azo dyes, etc., can solve the problems of high battery cost and complicated purification, and achieve the effect of strong power supply performance, high molar absorption coefficient, and inhibition of electronic recombination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
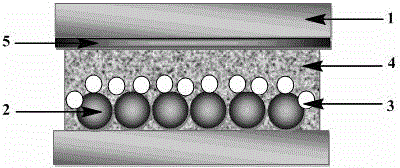
Image
Examples
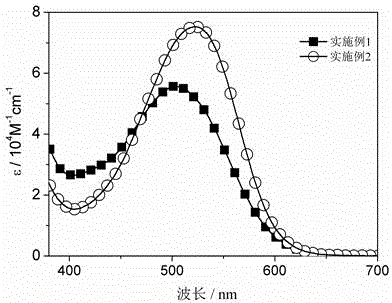
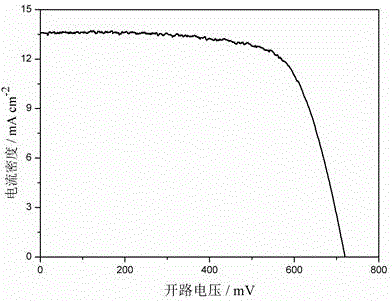
Embodiment 1
[0027] A preparation method of the triphenylamine-thiophene organic dyes, using aryl bromoaldehyde, tetrakistriphenylphosphine palladium, potassium carbonate and alkoxy substituted triphenylamine borate as raw materials, followed by Suzuki coupling Reaction and Knoevenagel condensation reaction to obtain the target product, the steps are as follows:
[0028] 1) Under the condition of nitrogen protection, in a 100 mL two-necked bottle, add 362 mg of hexyloxy-substituted triphenylamine borolipid (0.5 mmol), 109 mg (0.4 mmol) of bithiophene bromoaldehyde, and 29 mg of tetrasan Phenyl phosphorus (0.025 mmol), potassium carbonate 345 mg (2.5 mmol, 2M) and 30 mL of ethylene glycol dimethyl ether were mixed uniformly, and the obtained mixture was heated to reflux for 8 hours, and the obtained reaction liquid a was cooled to After room temperature, add 15 mL of water to quench, extract with ethyl acetate, separate liquids, dry the organic phase with anhydrous magnesium sulfate, distil...
Embodiment 2
[0040] A preparation method of the triphenylamine-thiophene organic dyes, using aryl bromoaldehyde, tetrakistriphenylphosphine palladium, potassium carbonate and alkoxy substituted triphenylamine borate as raw materials, followed by Suzuki coupling Reaction and Knoevenagel condensation reaction to obtain the target product, the steps are as follows:
[0041] 1) Under the condition of nitrogen protection, in a 100 mL two-necked bottle, add 362 mg of hexyloxy-substituted triphenylamine borolipid (0.5 mmol), 148 mg (0.4 mmol) of dithiophene pyrrole bromaldehyde, and 29 mg of tetra Triphenylphosphine (0.025 mmol), 345 mg of potassium carbonate (2.5 mmol, 2M) and 30 mL of ethylene glycol dimethyl ether were mixed uniformly, and the obtained mixture was heated to reflux for 8 hours, and the obtained reaction liquid a was cooled After reaching room temperature, add 15 mL of water to quench, extract with ethyl acetate, separate the liquids, dry the organic phase with anhydrous magnesi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com