Stenhouse donor-receptor adduct of Meldrum's acid-activated furan and 3-pyridylethylamine, and synthesis method thereof
A synthetic method, technology of pyridineethylamine, applied in chemical instruments and methods, organic chemistry, color-changing fluorescent materials, etc., can solve problems such as harsh reaction conditions, poor selectivity, and rare raw materials, and achieve easy purification, easy acquisition, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Aromatic compound I of the present invention, its molecular structure is as follows:
[0042]
[0043] The preparation method of the furan compound of present embodiment 1 comprises the following steps:
[0044] In the two-necked bottle, according to the molar ratio of 2-furyl aldehyde: cycloisopropylidene malonate=1:1.1, 2-furyl aldehyde and cycloisopropylidene malonate were mixed first, and then deionized water was added as Solvent, reacted at 75°C for 3h. After the reaction, add CH 2 Cl 2 Extract crude product, CH 2 Cl 2 Extract water (25ml*3) then concentrate CH 2 Cl 2 Phase, add saturated NH successively 4 Cl solution, saturated NaCl solution, saturated NaHSO 4 , saturated NaHCO 3 solution, for CH 2 Cl 2 phase was washed, and then a small amount of water in the aqueous phase was dried with anhydrous magnesium sulfate, rotary evaporated, and vacuumized by an oil pump to obtain a yellow blocky solid (to be stored in nitrogen). It was characterized by N...
Embodiment 2
[0046] The target compound, its molecular structure is shown in the following formula:
[0047]
[0048] The preparation method of the DASA compound of present embodiment 2 comprises the following steps:
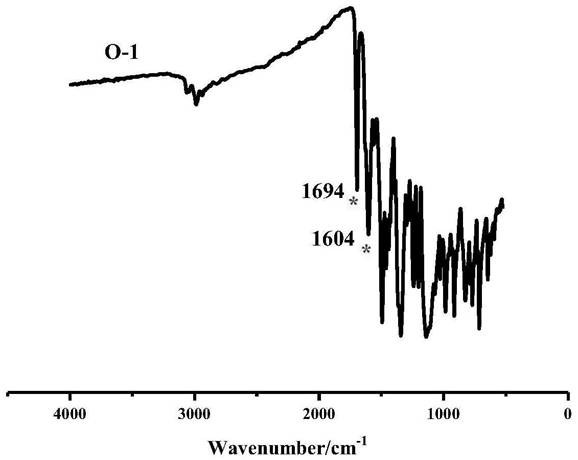
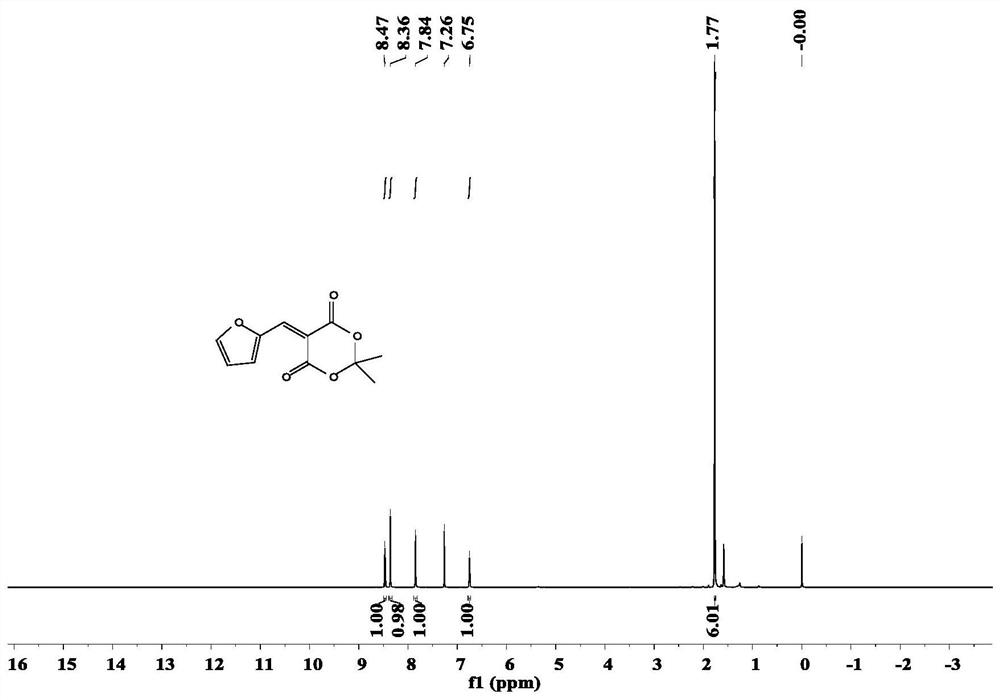
[0049] Under nitrogen protection, in a one-necked flask according to the molar ratio 3-Pyridylethylamine mix. The mixture was added to a tetrahydrofuran solution, stirred at 23°C for 10 minutes, and then cooled at 0°C for 20 minutes. The reaction mixture was then filtered to collect the precipitated solid, which was washed with cold diethyl ether and dried in vacuo to give a red solid. NMR and IR characterization were performed on it, and the results were as follows: figure 2 , image 3 shown.
Embodiment 3
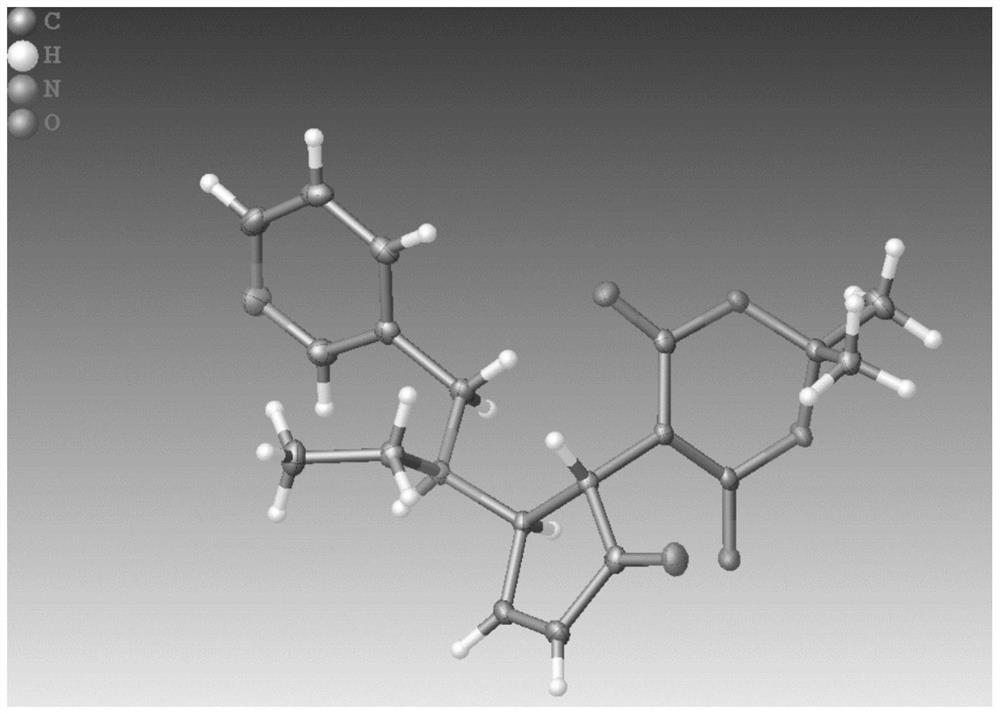
[0051]Compound II was recrystallized in methanol and allowed to stand for 1-2 days to obtain Compound III. It is characterized by XRD, such as Figure 4 shown.
[0052] The crystal data obtained after recrystallization of the red solid are shown in Table 1 below.
[0053]
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com