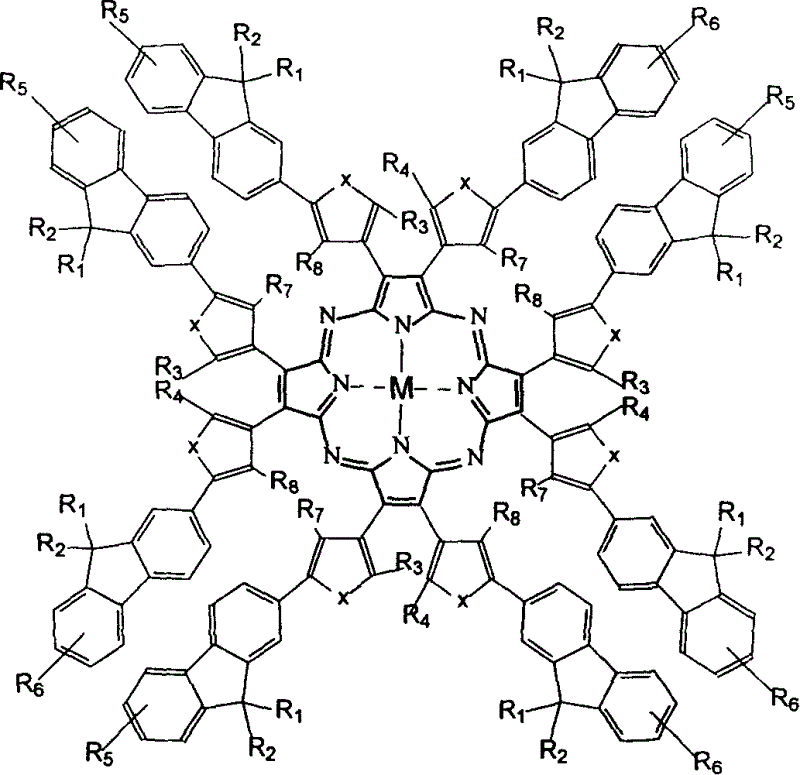
Metal aza porphyrin compound containing fluorene diaryl ethylene and its preparation method and application
A diarylethene compound technology, which is applied in the field of diarylethene metal azaporphyrin compounds and its preparation, can solve the problems of destroying information, authenticity and integrity of ring-destroying information records, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Using Method 1 to synthesize target compound M 1
[0051] The synthesis of intermediate: In a 100mL single-necked flask, 3mmol of 2-halo-9,9'-dioctylfluorene, 1,2-dianitrile-1,2-bis(2-methyl-5-halo -3-thiophene)-ethylene 1.3mmol, metal magnesium or sodium; use tetrahydrofuran or ether as solvent, and add a certain catalyst. The system was replaced with argon in vacuum three times, and refluxed overnight in an argon atmosphere, protected from light. Pour into water, separate the layers, extract the inorganic phase with ether, combine the organic layers, and concentrate. Use petroleum ether and dichloromethane as eluents to pass through a silica gel column. The obtained light yellow solid is the intermediate with a yield of 64%. MS(m / e): 1047.8. 1 H-NMR (400MHz, CDCl 3 , Ppm): 0.86 (t, 12H, -CH 3 ), 2.23(s, 6H, -CH 3 ), 6.62(s, 2H, -CH-), 1.02-1.23(m, 48H, -CH 2 -), 1.95(m, 8H, -CH 2 -), 7.25-7.36 (m, 4H, Ar) 7.43-7.48 (m, 6H, Ar) 7.79-7.84 (m, 4H, Ar).
[0052] ...
Embodiment 2
[0055] Example 2 Using method 2 to synthesize target compound M 1
[0056] Under the protection of argon, add 20mmol of metallic magnesium to 100mL of dry isopropanol and reflux for 24 hours; cool to room temperature, and then add 2mmol of 1,2-diconitrile-1,2-bis(2-methyl- The 5-halo-3-thiophene)-ethylene compound was refluxed for 36 hours. The reaction was carried out under protection from light and argon. After the reaction was completed, it was cooled, the solvent was removed under reduced pressure, washed, dried, filtered, concentrated, Silica gel column to obtain pure octahalodithiophene vinyl metal azaporphyrin compound. The yield is about 55%.
[0057]
[0058] Then, the azaporphyrin is reacted with 2-halo-9,9'-dioctylfluorene in a polar solvent under the action of a catalyst, and refluxed overnight in an argon atmosphere, protected from light. Cool to room temperature, filter, concentrate, use chloroform or dichloromethane and petroleum ether as the eluent, and pass thr...
Embodiment 3
[0059] Example 3 Photochromic color development method
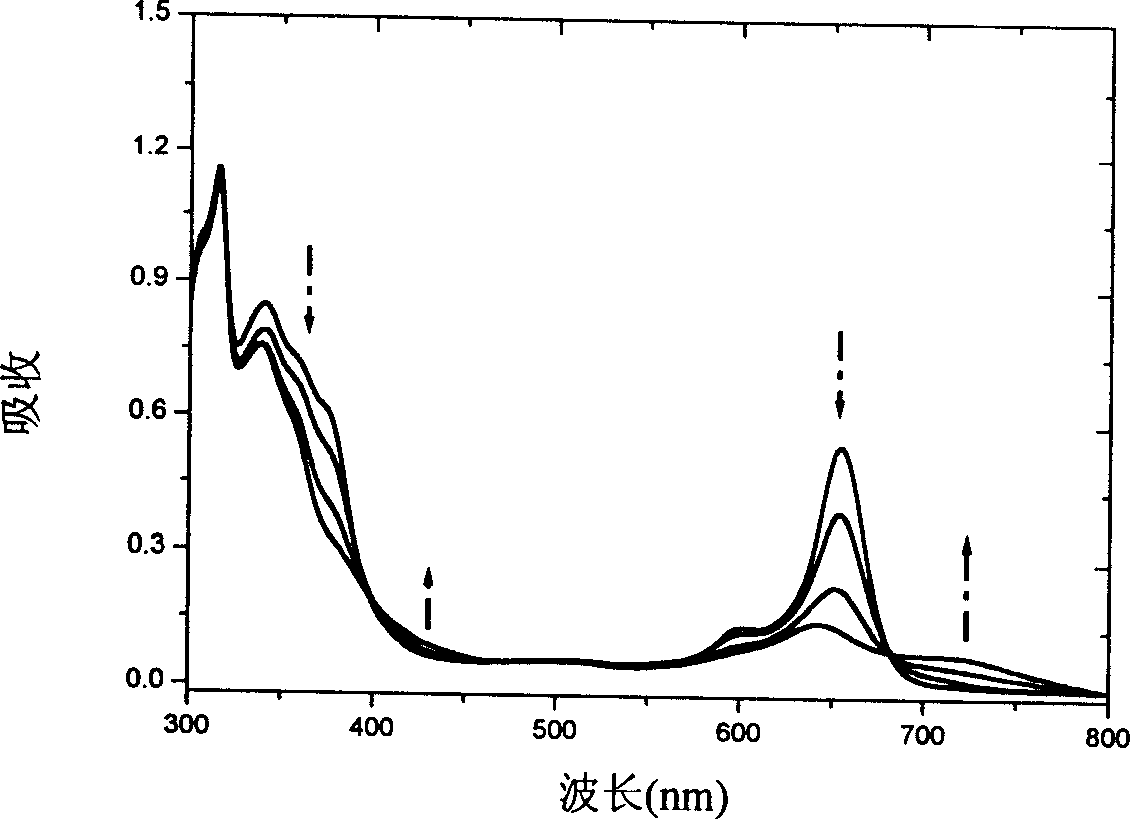
[0060] Before being irradiated with ultraviolet light, the target compound in Example 1 or 2-the azaporphyrin compound has three strong absorption peaks, located at 653nm, 340nm and 315nm, and a weak broad absorption band, located between 400-450nm between. When its methylene chloride solution or chloroform is irradiated with ultraviolet light with a wavelength of 300-365nm, its absorption spectrum changes significantly. The figure below shows the dynamic change spectrum obtained at different intervals. We can clearly see that the strong absorption peak at 653nm gradually weakened, while a new absorption peak appeared at about 720nm and gradually increased. After about 30 minutes of irradiation, the system reached a light-stable state, and a set of isoabsorption points appeared at 320, 396, 471, 570, and 683 nm. The color of the solution also changed from blue-green to dark green. The new absorption peak at 720nm is due to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com