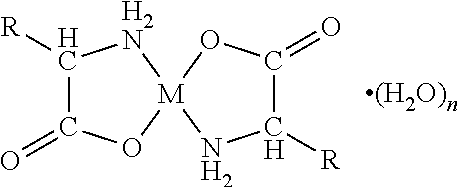
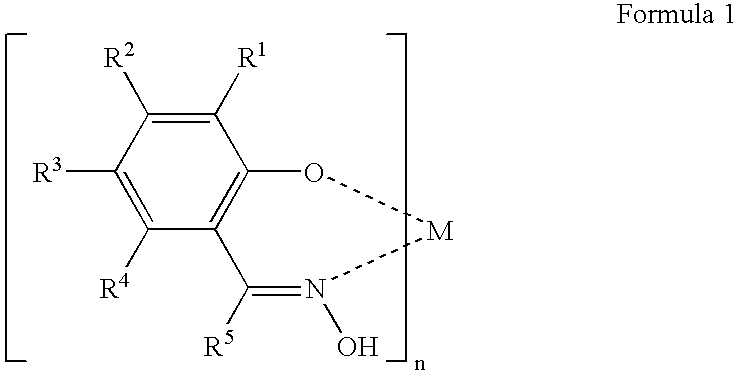
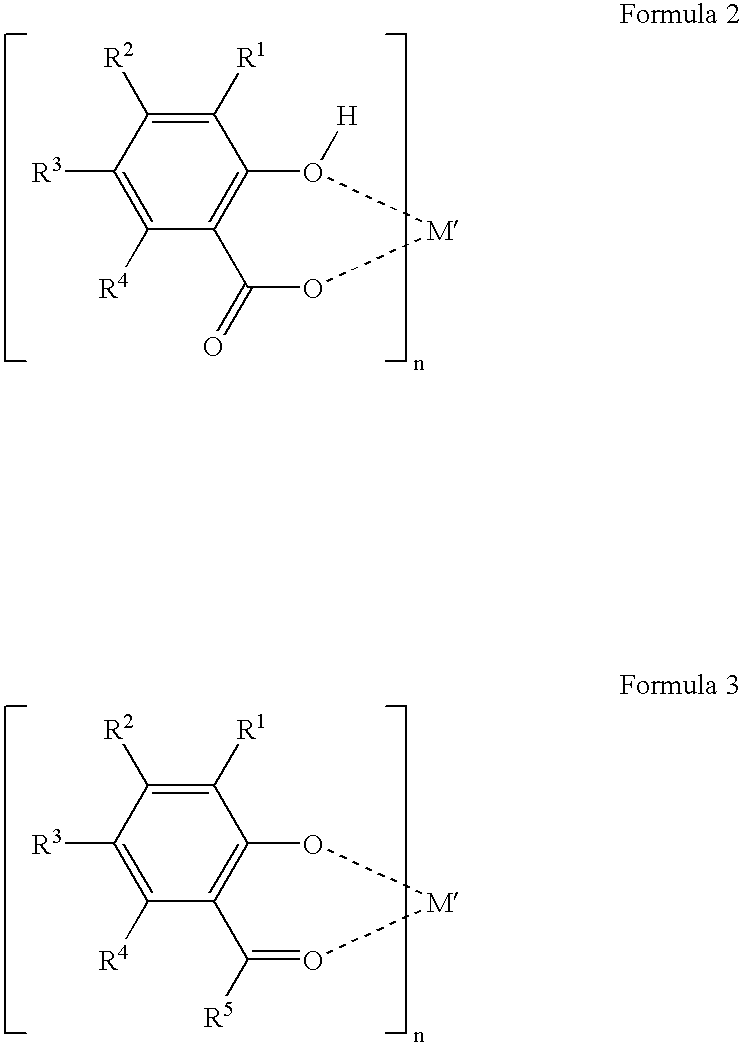
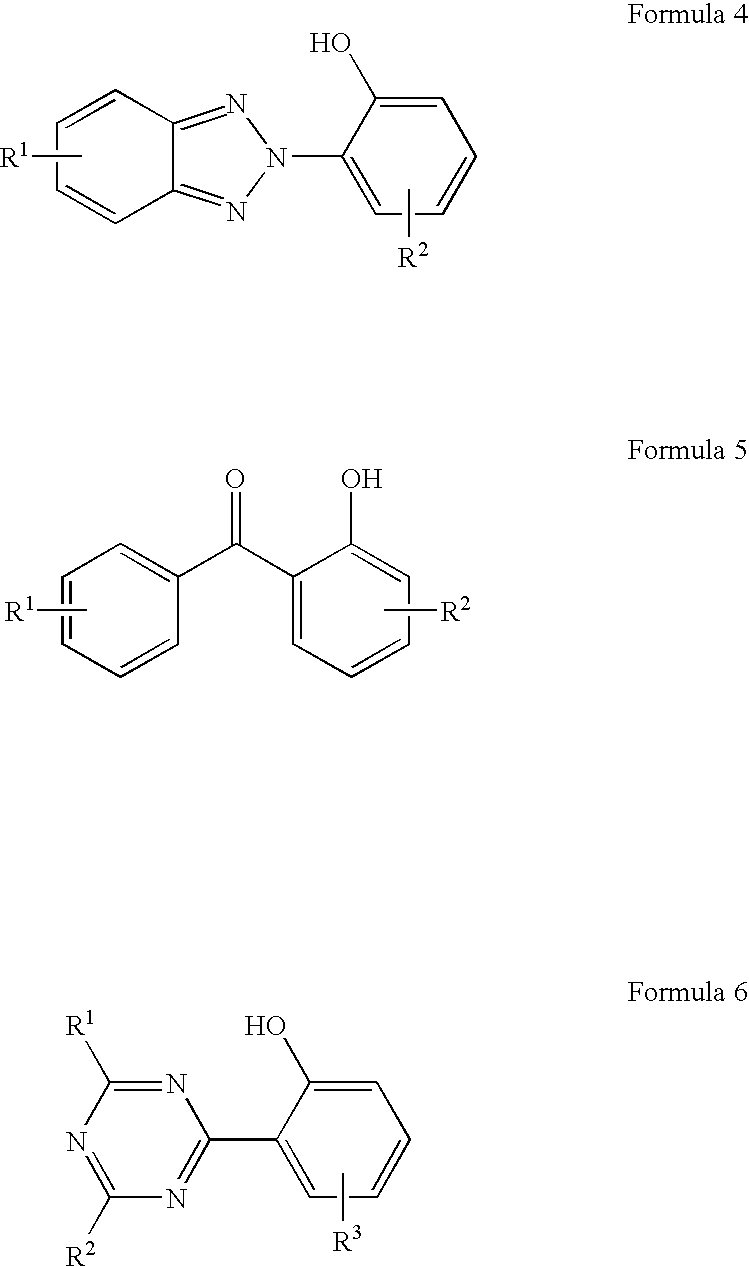
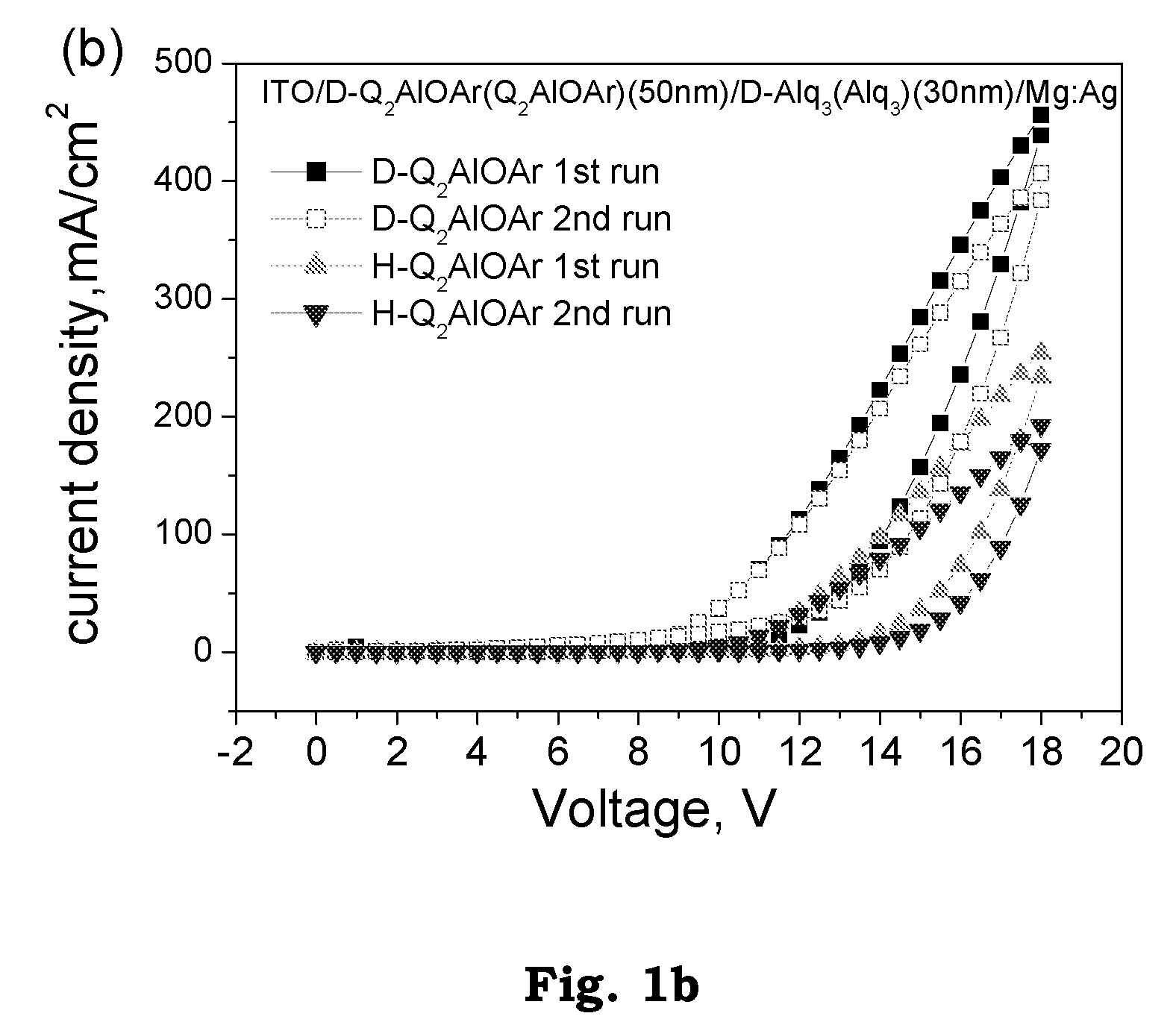
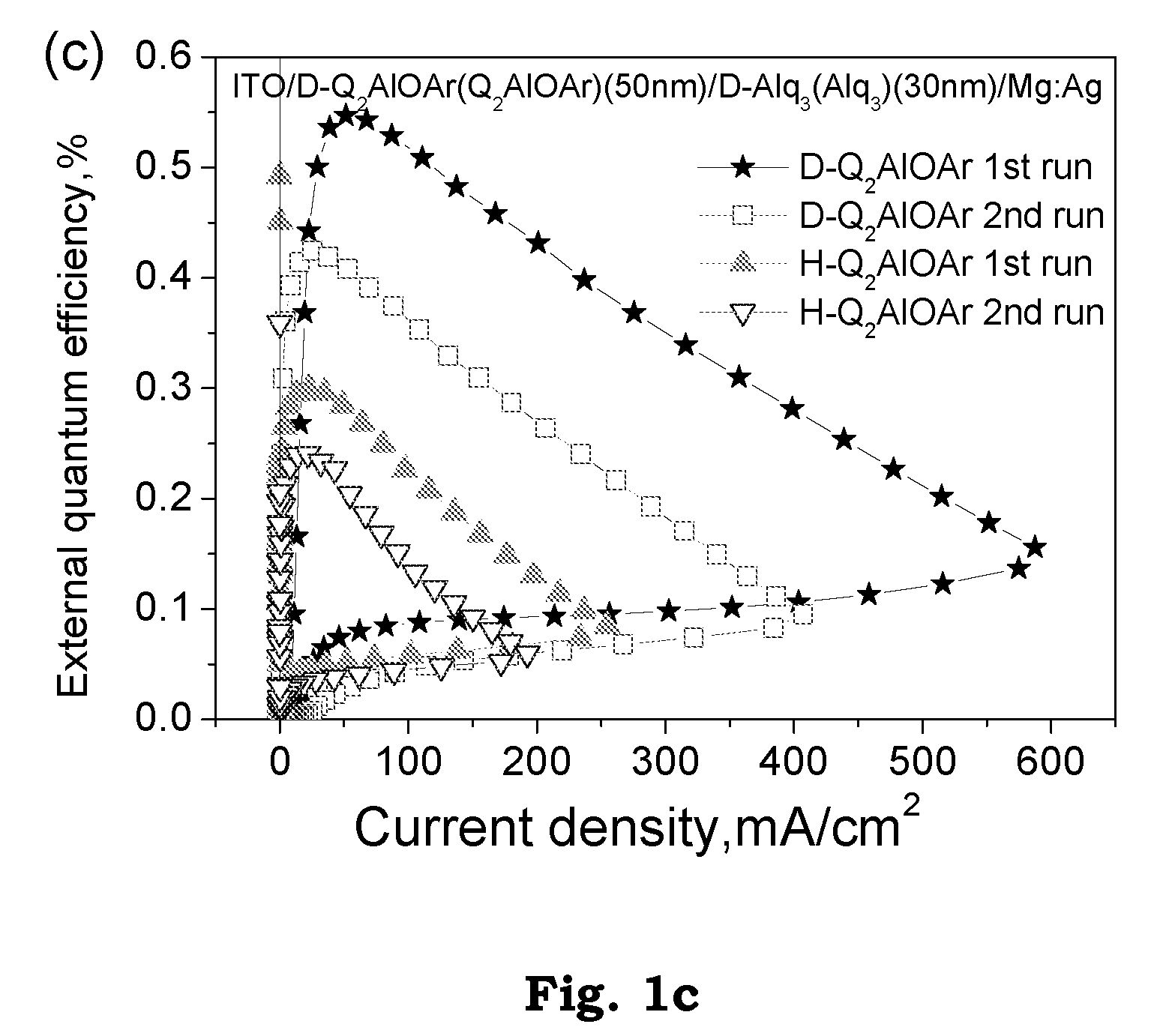
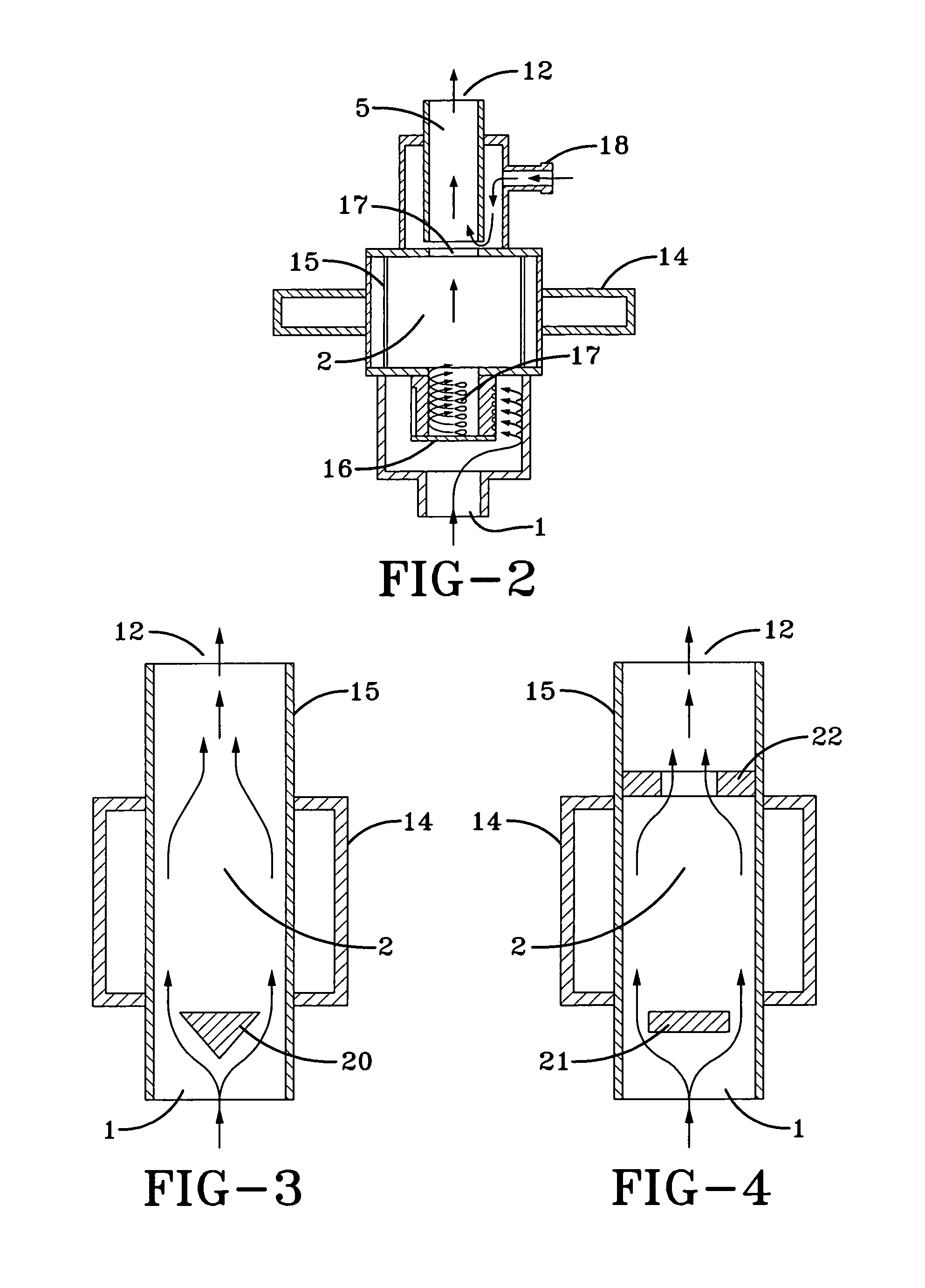
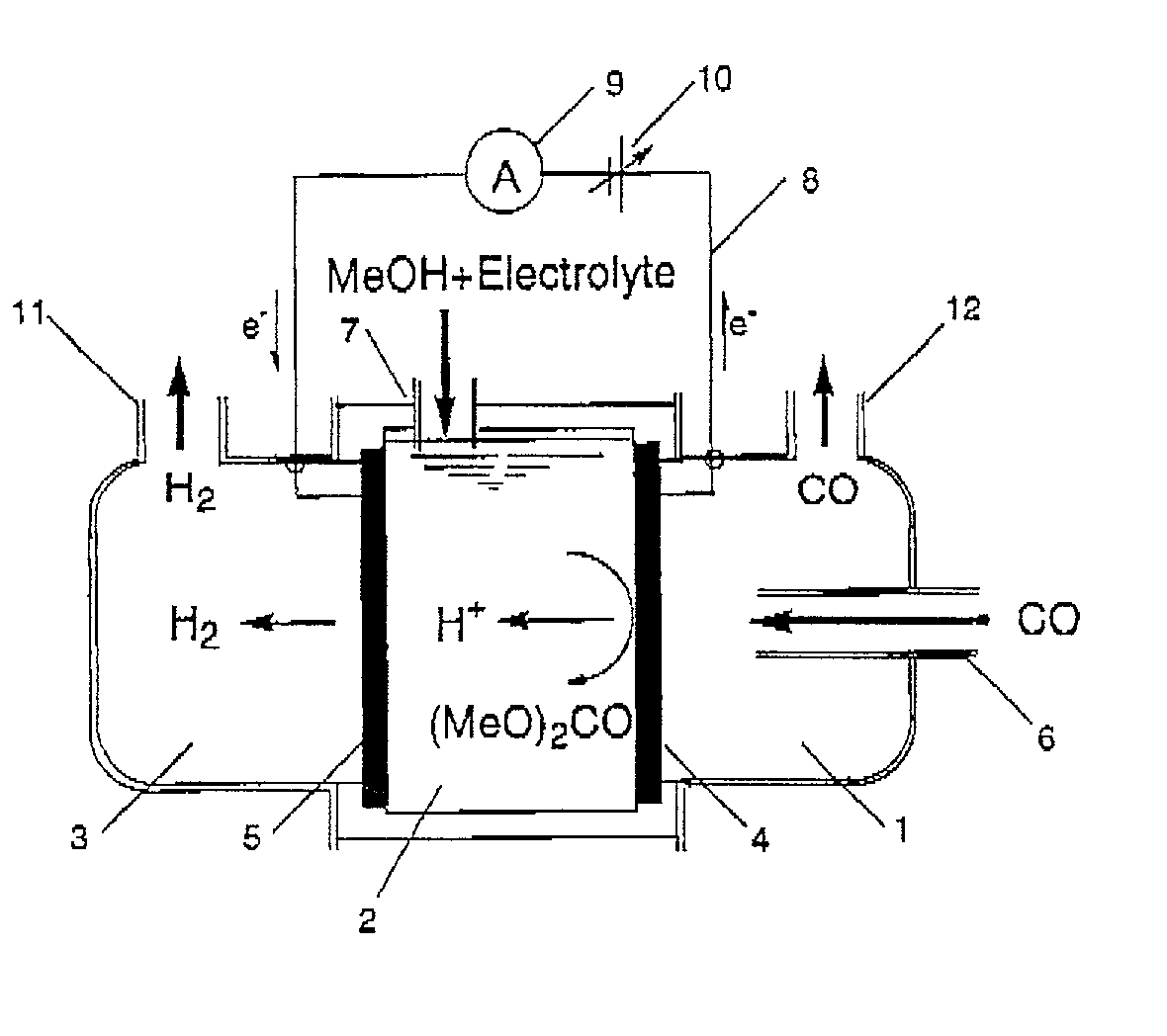
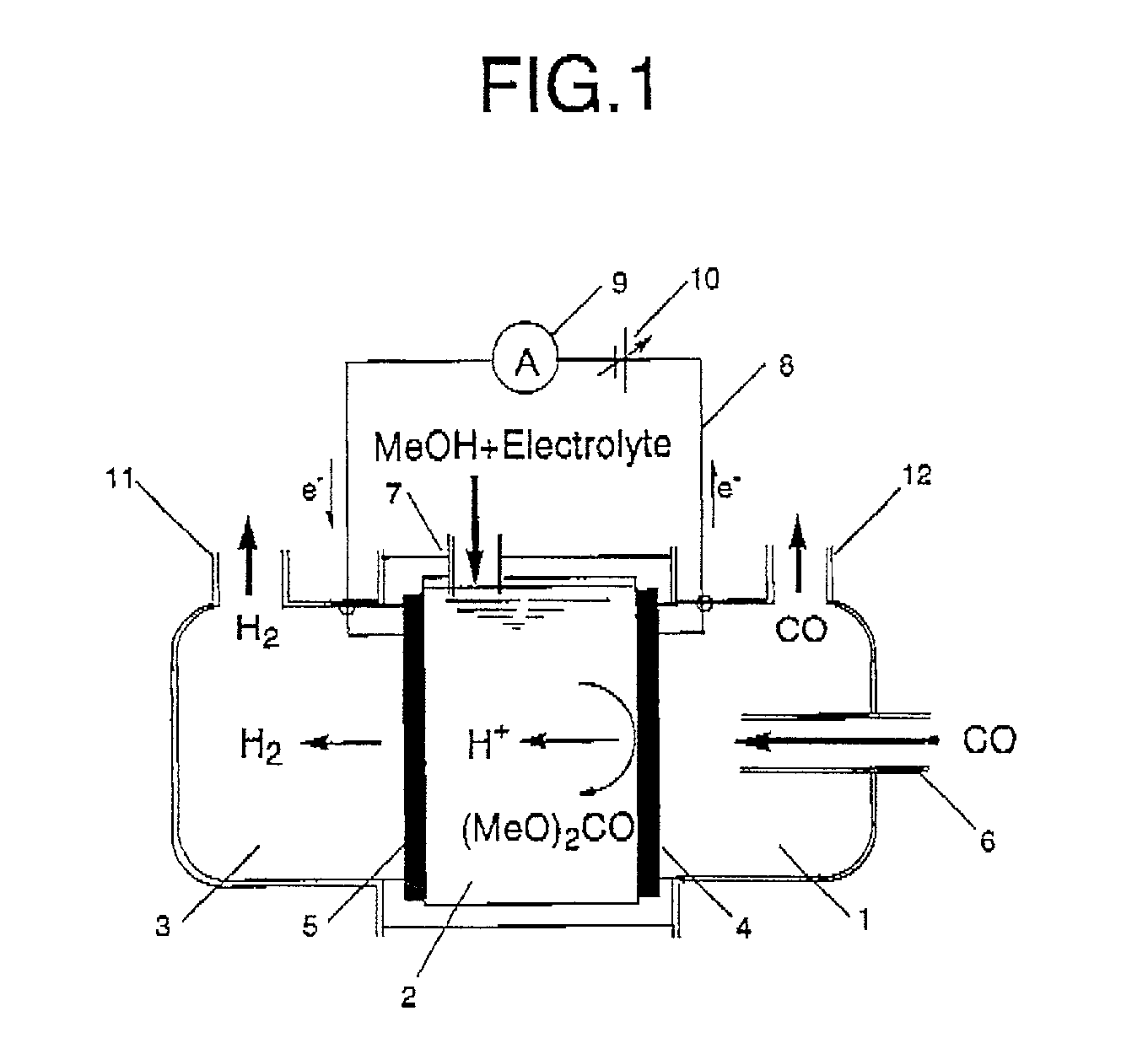
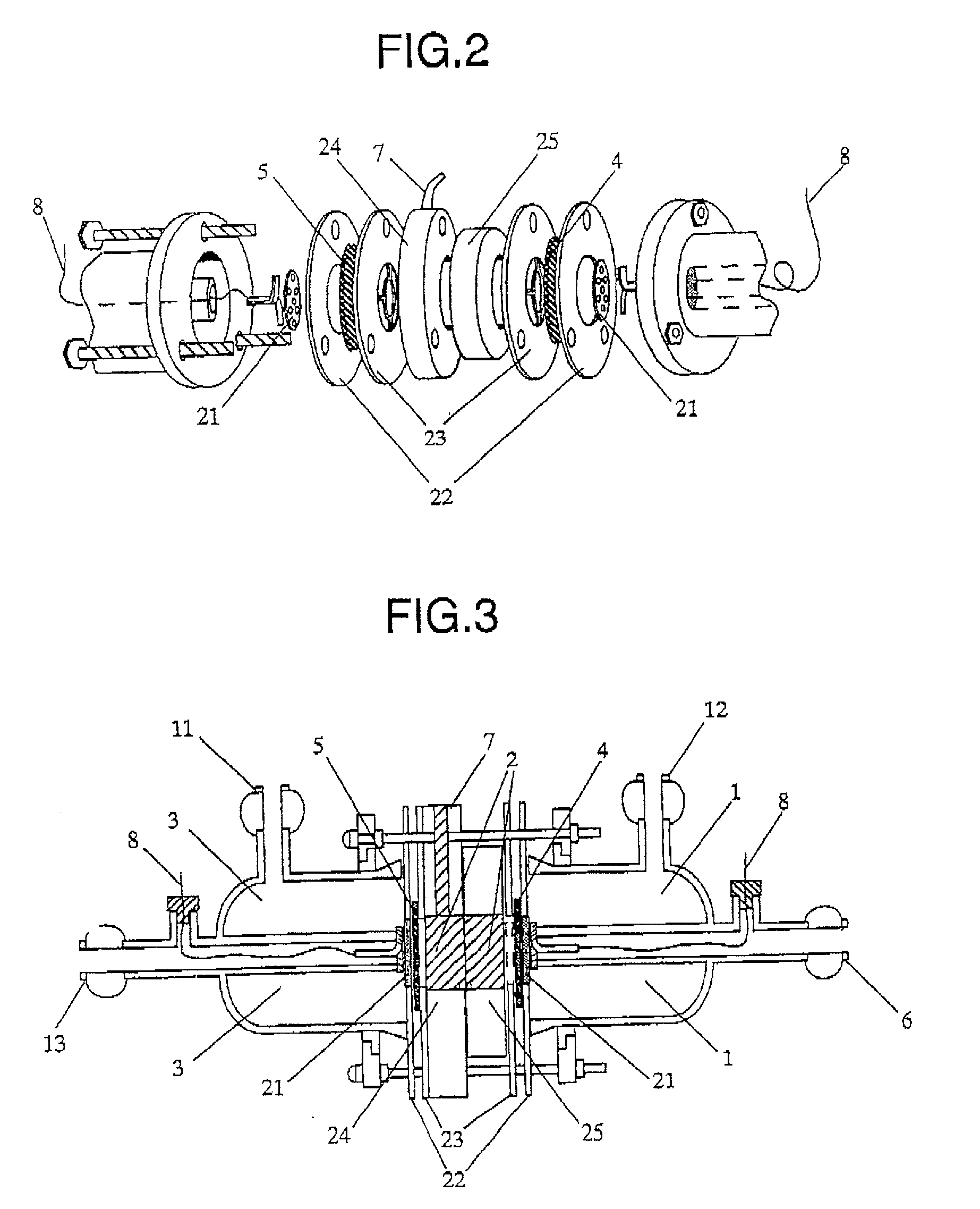
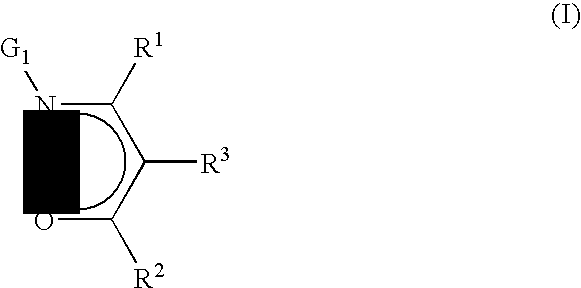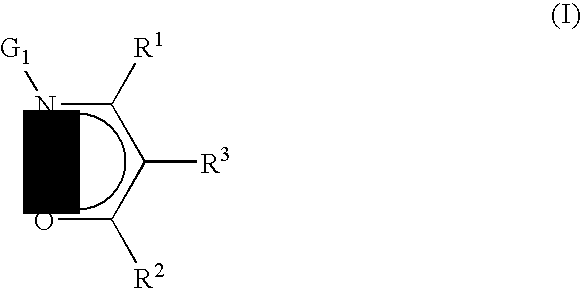Patents
Literature
260results about "Group 2/12 element organic compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Organoaminosilane precursors and methods for making and using same
Described herein are organoaminosilane precursors which can be used to deposit silicon containing films which contain silicon and methods for making these precursors. Also disclosed herein are deposition methods for making silicon-containing films or silicon containing films using the organoaminosilane precursors described herein. Also disclosed herein are the vessels that comprise the organoaminosilane precursors or a composition thereof that can be used, for example, to deliver the precursor to a reactor in order to deposit a silicon-containing film.
Owner:VERSUM MATERIALS US LLC
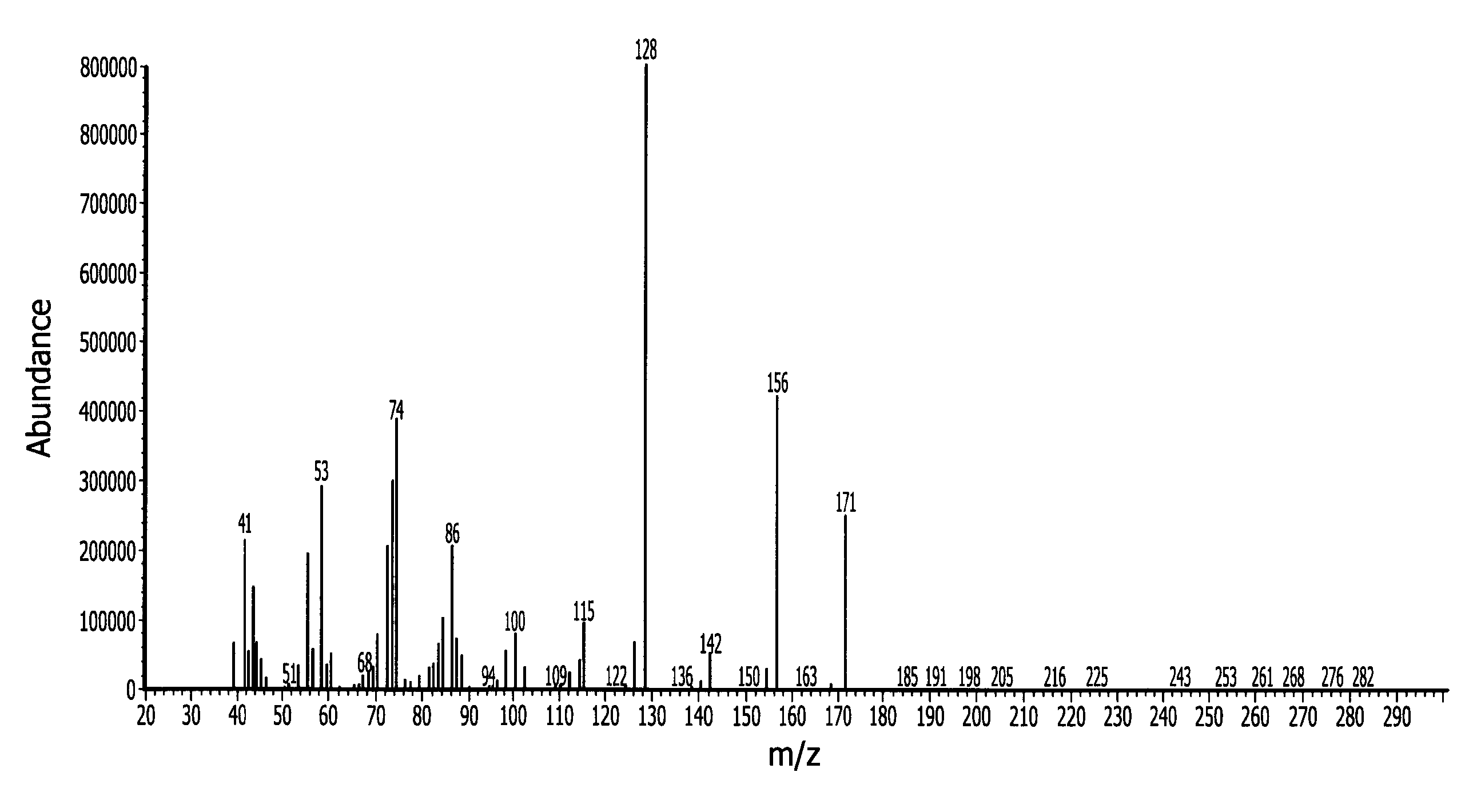
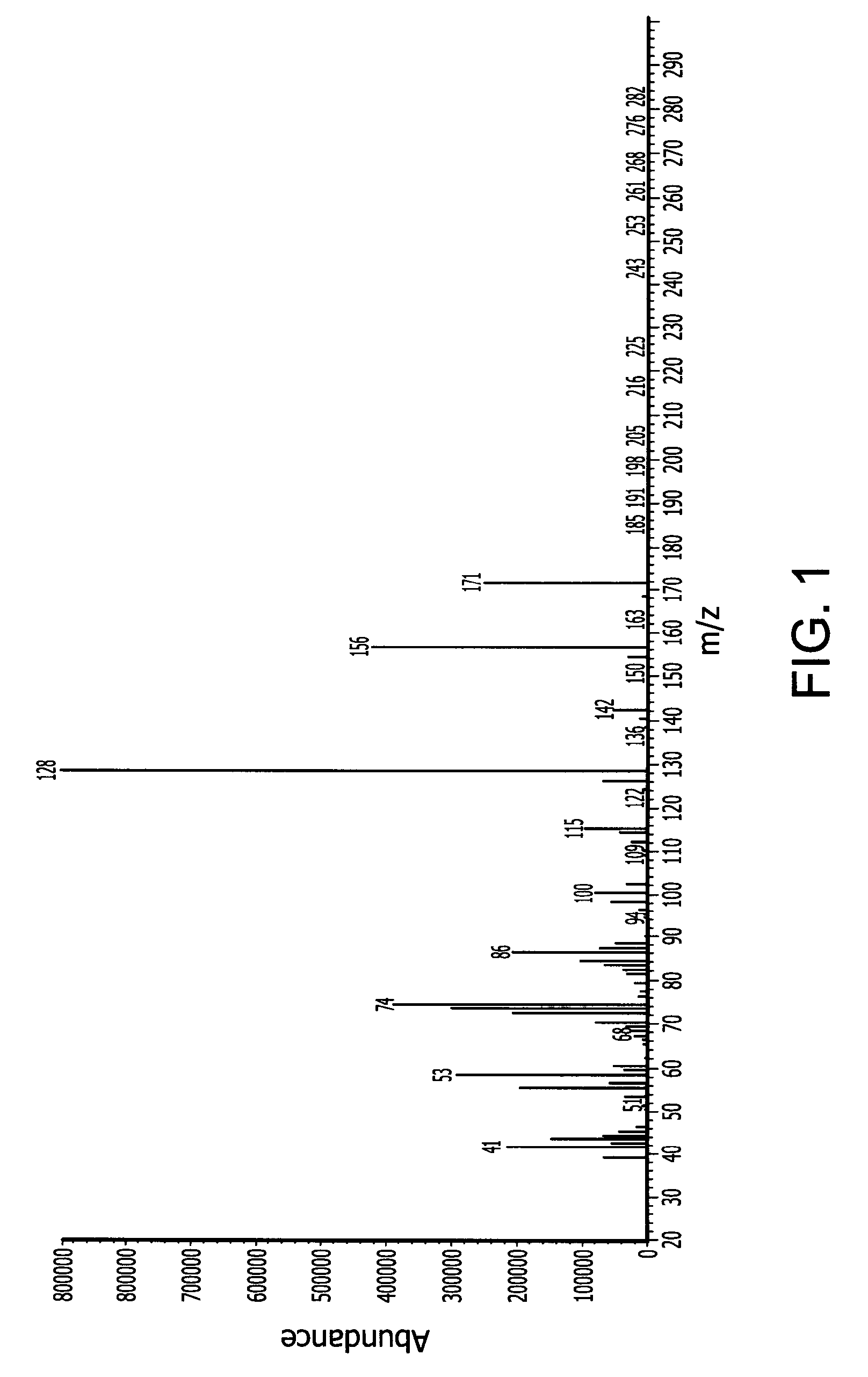
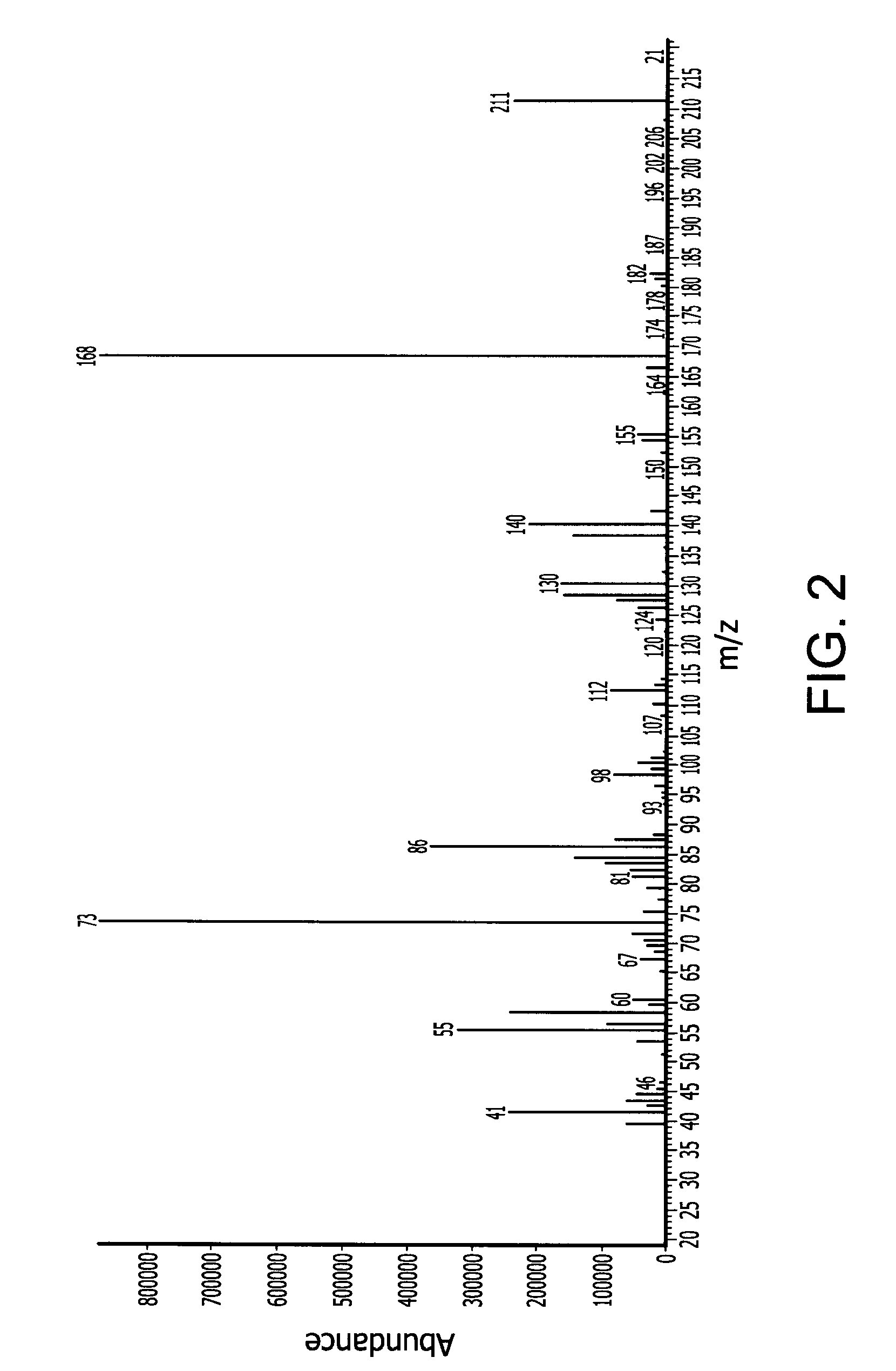
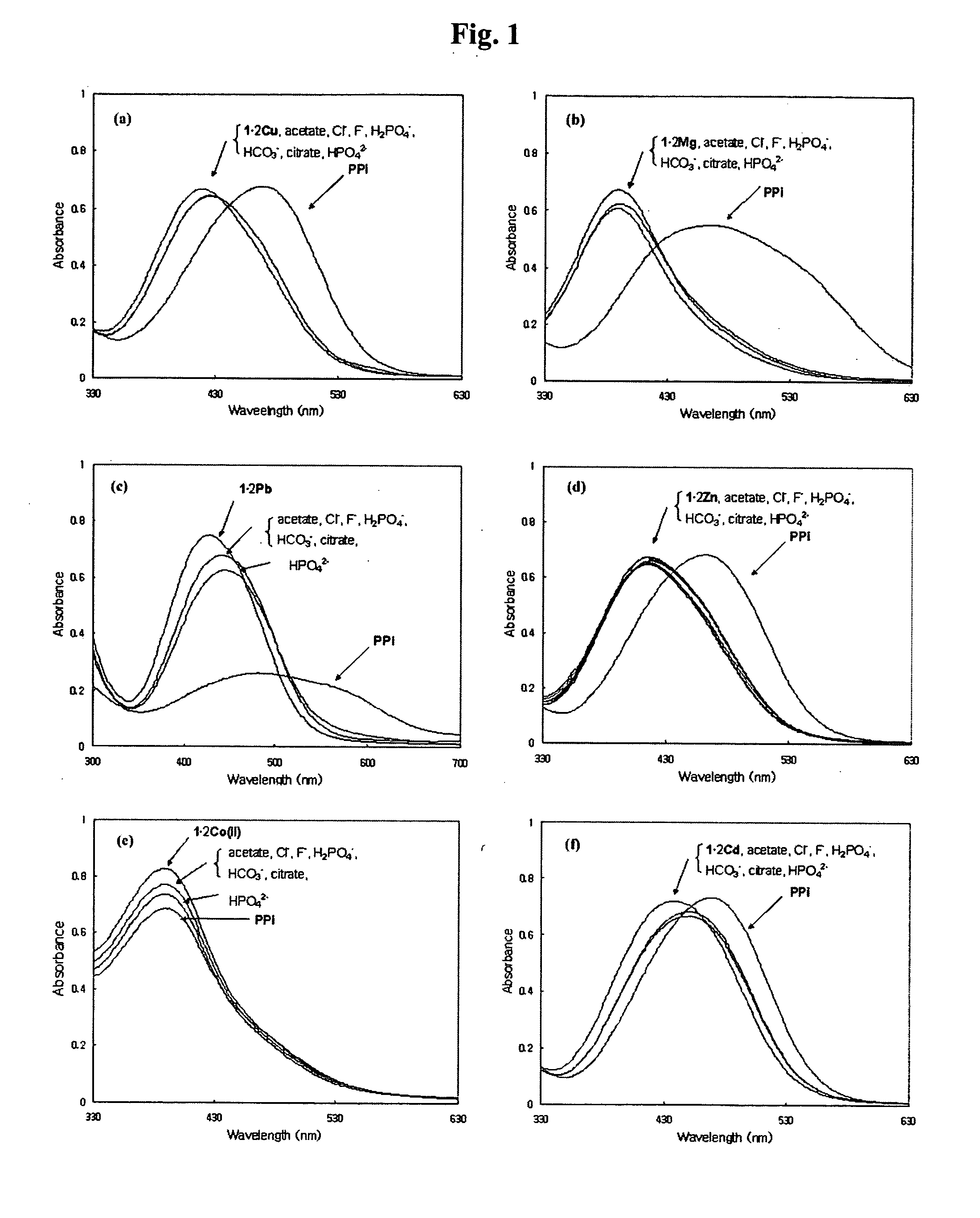
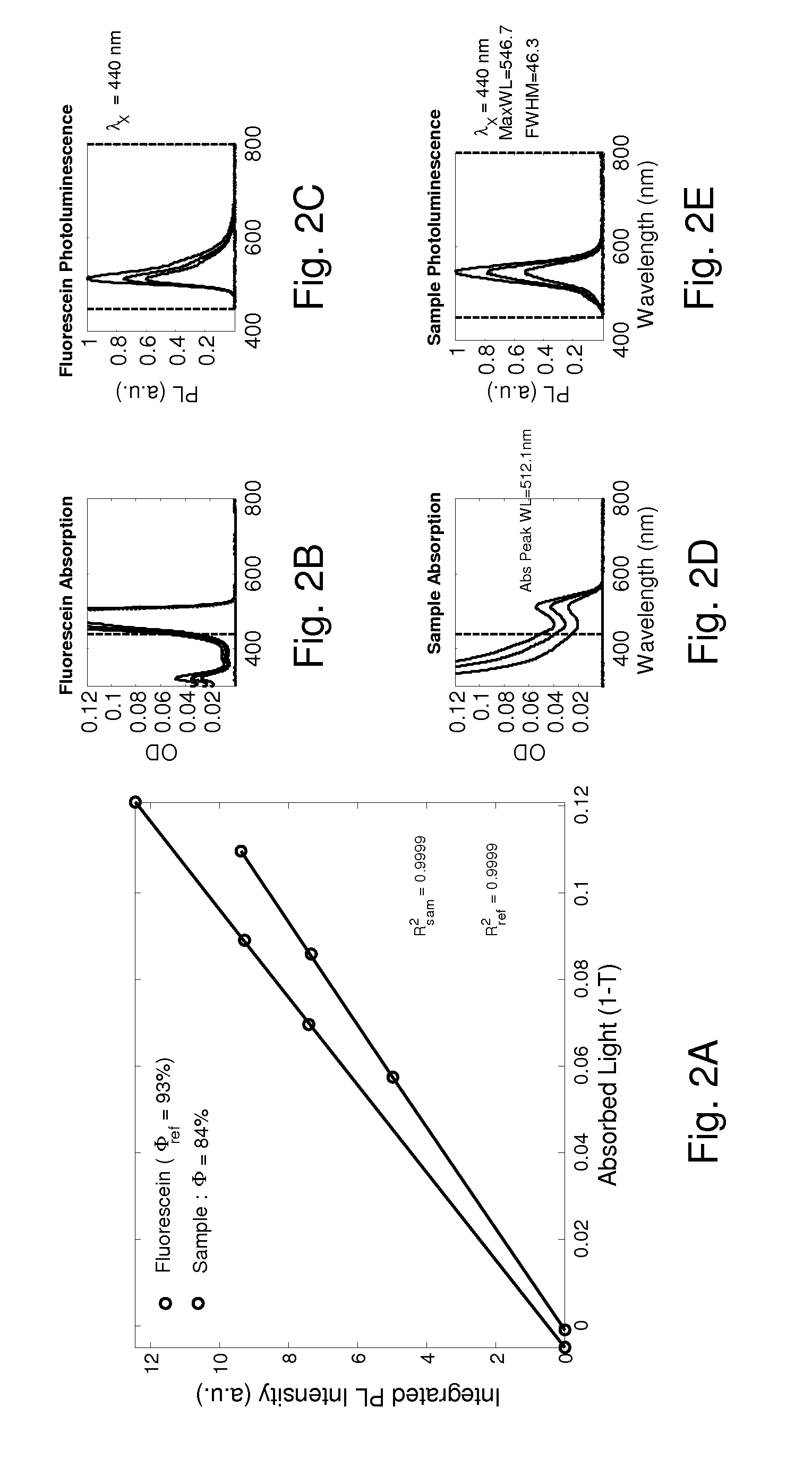
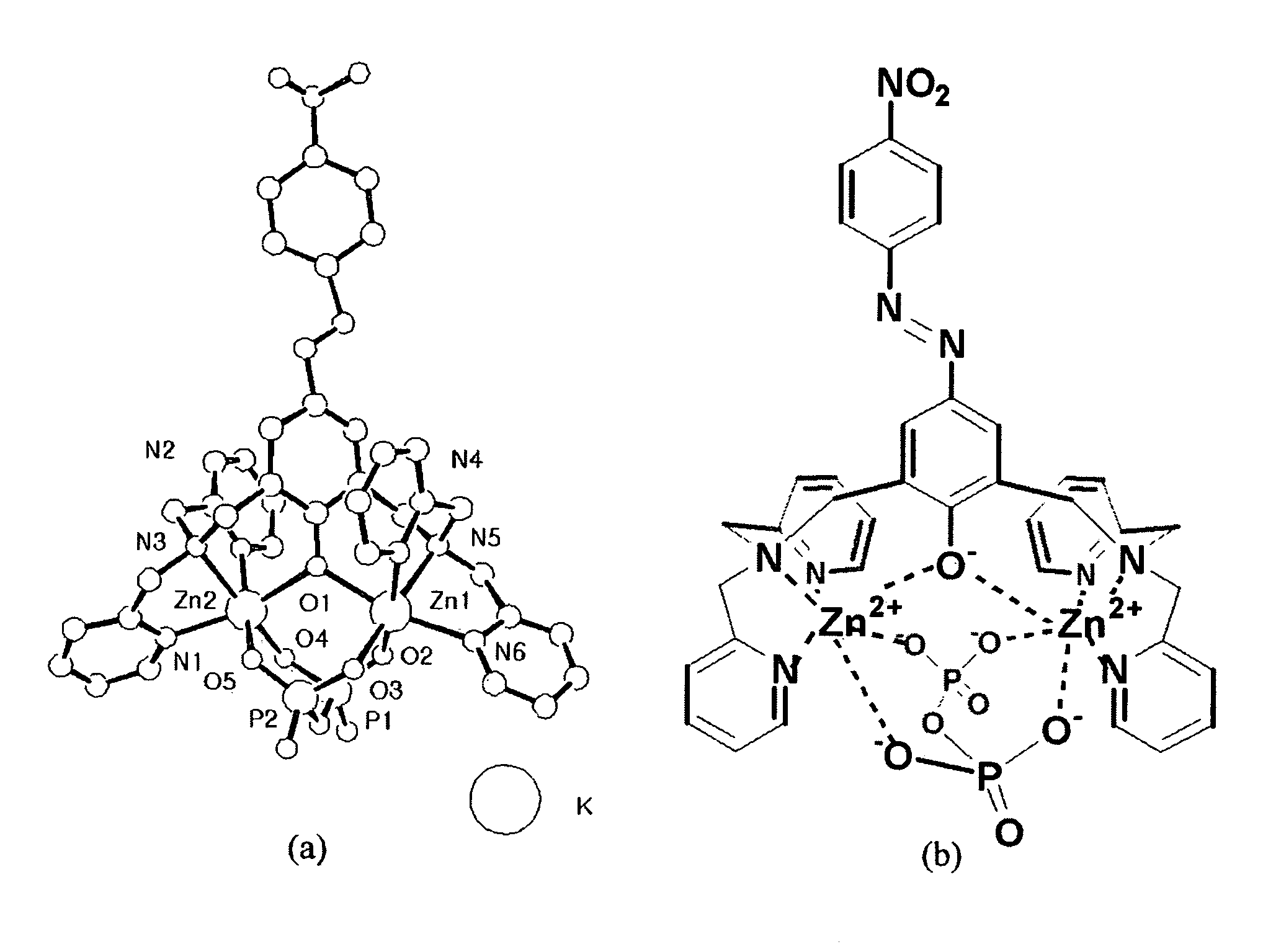
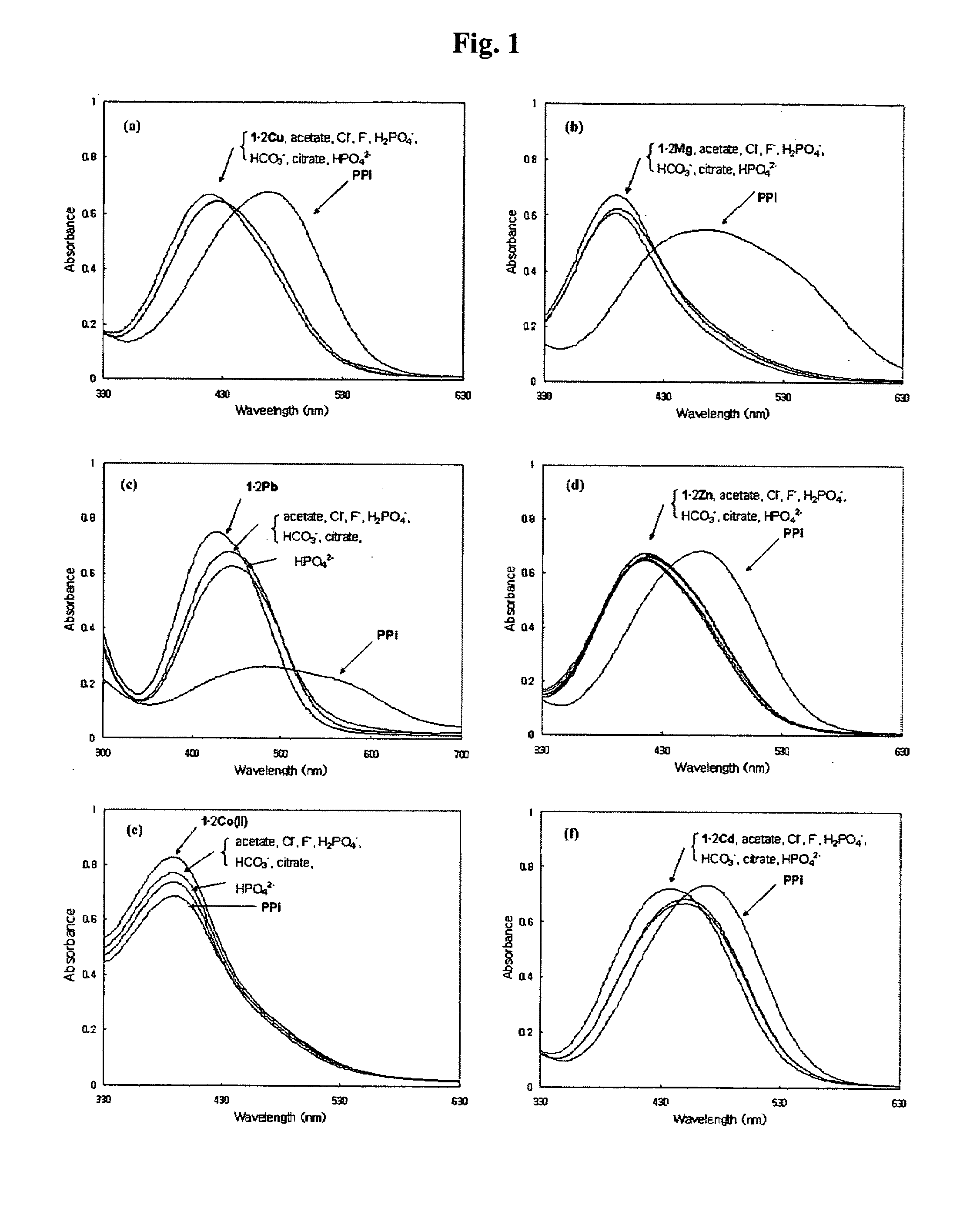
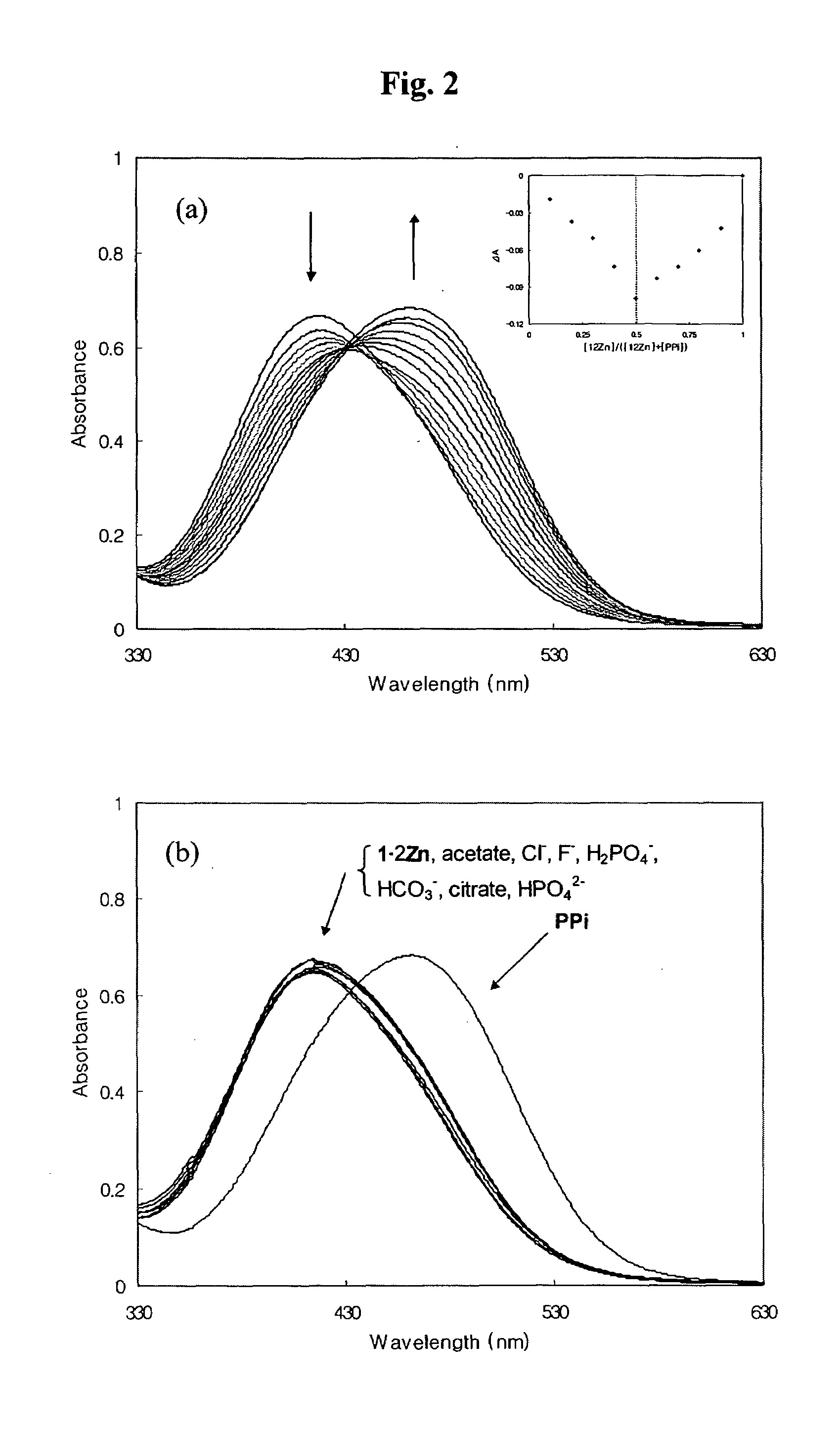
Novel dinuclear metal complex and pyrophosphate assay using the same
InactiveUS20050119497A1Increase electron densityEnhanced electron donationUltrasonic/sonic/infrasonic diagnosticsGroup 8/9/10/18 element organic compoundsElectronic densityPyrophosphate
A novel coordination complex formed by dinuclear metal complexation is provided. The complex is a dinuclear metal complex of a compound, wherein the compound comprises a conjugation ring system substituted with: a) an electron donating group selected from —OH, —SH and —NH2; b) an indicating group selected from a chromogenic group, a fluorescent group and an electrochemical group; and c) two binding auxiliary groups, in combination with the electron donating group each of which being coordinated with the metal to provide an anion bonding site, wherein as the complex binds to a anion, the coordination of the electron donating group with the metal is weakened and electron donation of the electron donating group to the conjugation ring system is reinforced such that the reinforced electron donation by the electron donating group is transferred through the conjugation ring system to the indicating group to produce an indicating signal concomitant with the change of its electronic density. The coordination complex shows high sensitivity and high selectivity for pyrophosphate over other anions in an aqueous solvent over a wide pH range. Therefore, the complex is useful for pyrophosphate assay as a pyrophosphate sensor.
Owner:SEOUL NAT UNIV FOUND
Method and Apparatus for Microwave Reduction of Organic Compounds
InactiveUS20070102279A1Maximum protectionElectrical coke oven heatingSolid waste disposalEngineeringVolumetric Mass Density
The invention described herein generally pertains to utilization of high power density microwave energy to reduce organic compounds to carbon and their constituents, primarily in a gaseous state. The process includes, but is not limited to, scrap tires, plastics, asphalt roofing shingles, computer waste, medical waste, municipal solid waste, construction waste, shale oil, and PCB / PAH / HCB-laden materials. The process includes the steps of feeding organic material into a microwave applicator and exposing the material to microwave energy fed from at least two linear polarized sources in non-parallel alignment to each other, and collecting the material. The at least two sources of microwave energy are from a bifurcated waveguide assembly, whose outputs are perpendicular to each other and fed through waveguide of proper impedance, such that the microwave sources are physically and electrically 90° out of phase to each other. The microwave frequency is between 894 and 1000 MHz, preferably approximately 915 MHz.
Owner:NOVAK JUDITH
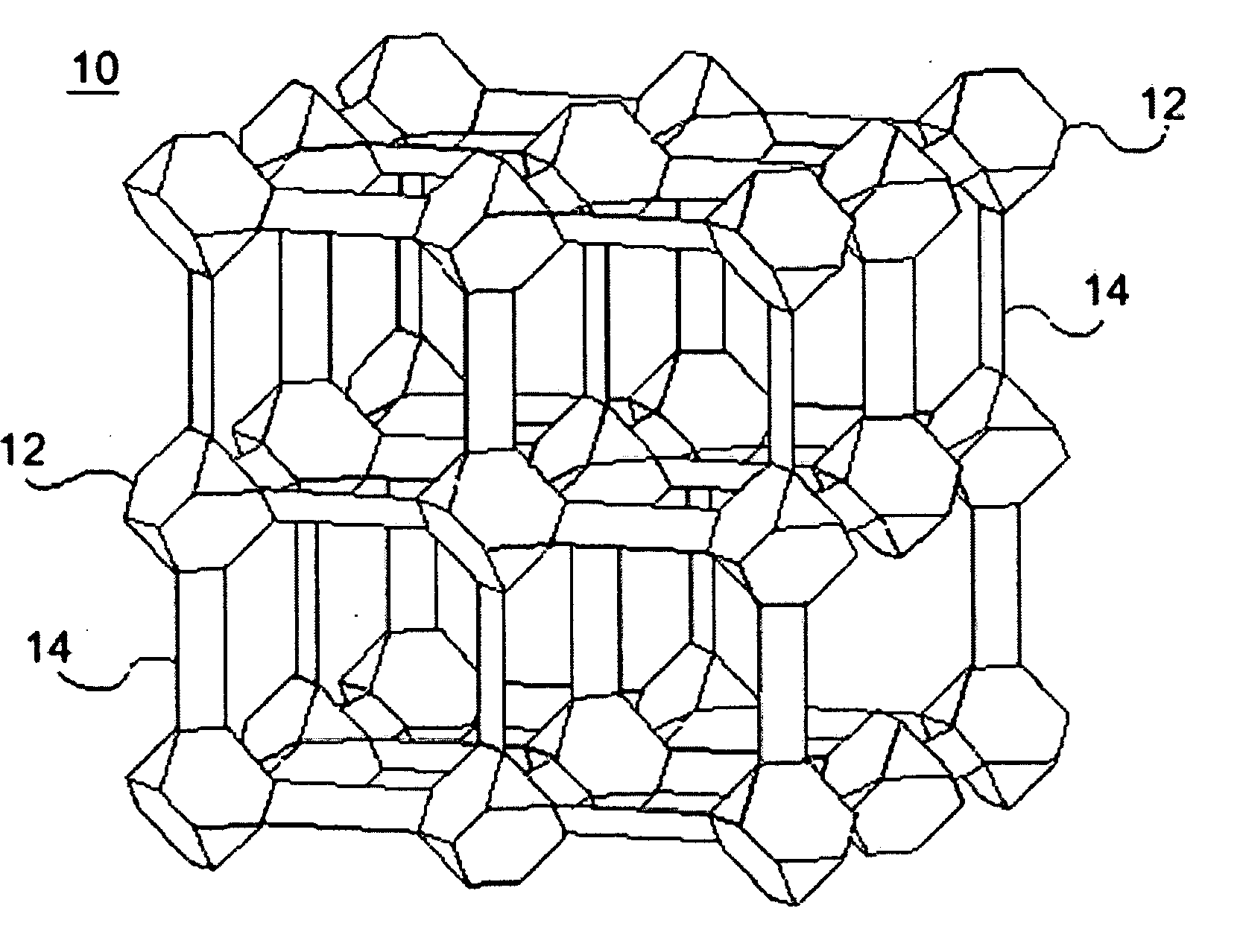
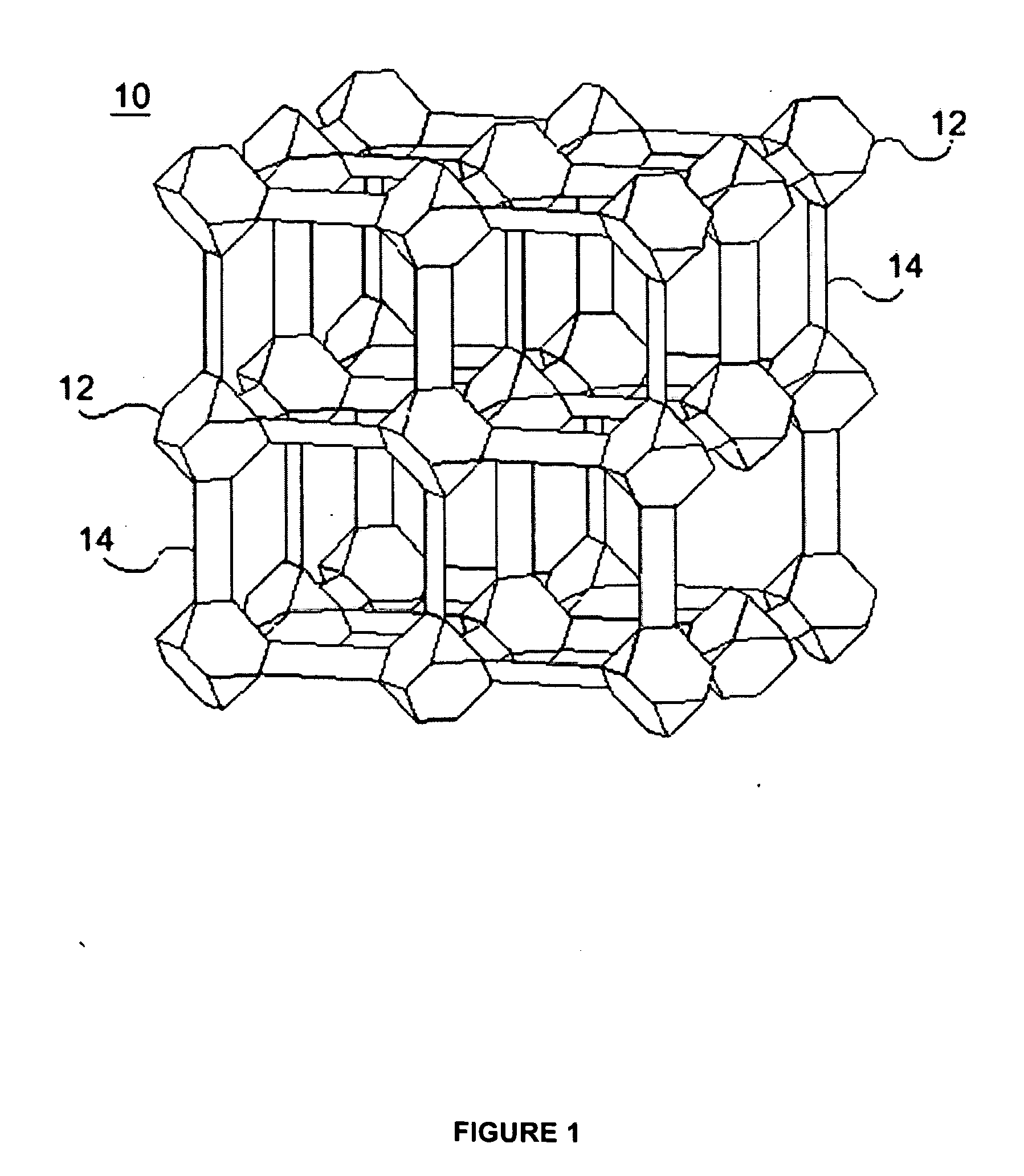
Zeolite-like metal organic frameworks (ZMOFS): modular approach to the synthesis of organic-inorganic hybrid porous materials having a zeolite like topology
InactiveUS20060287190A1Molecular sieve catalystsOther chemical processesMetal-organic frameworkModularity
The subject invention pertains to metal organic frameworks (MOF) having zeolite-net-like topology, their methods of use, and their modes of synthesis. The ZMOFs are produced by combining predesigned tetrahedral building, generated in situ using heterochelation, with polyfunctional ligands that have the commensurate angle and the required donor groups for the chelation. Each molecular building block is contrasted of a single metal ion and ligands with both heterochelation functionality and bridging functionality. Advantageously, zeolite-net-like MOFs of the subject invention are porous and contain large functional cavities, which is useful for encapsulating large molecules.
Owner:UNIV OF SOUTH FLORIDA
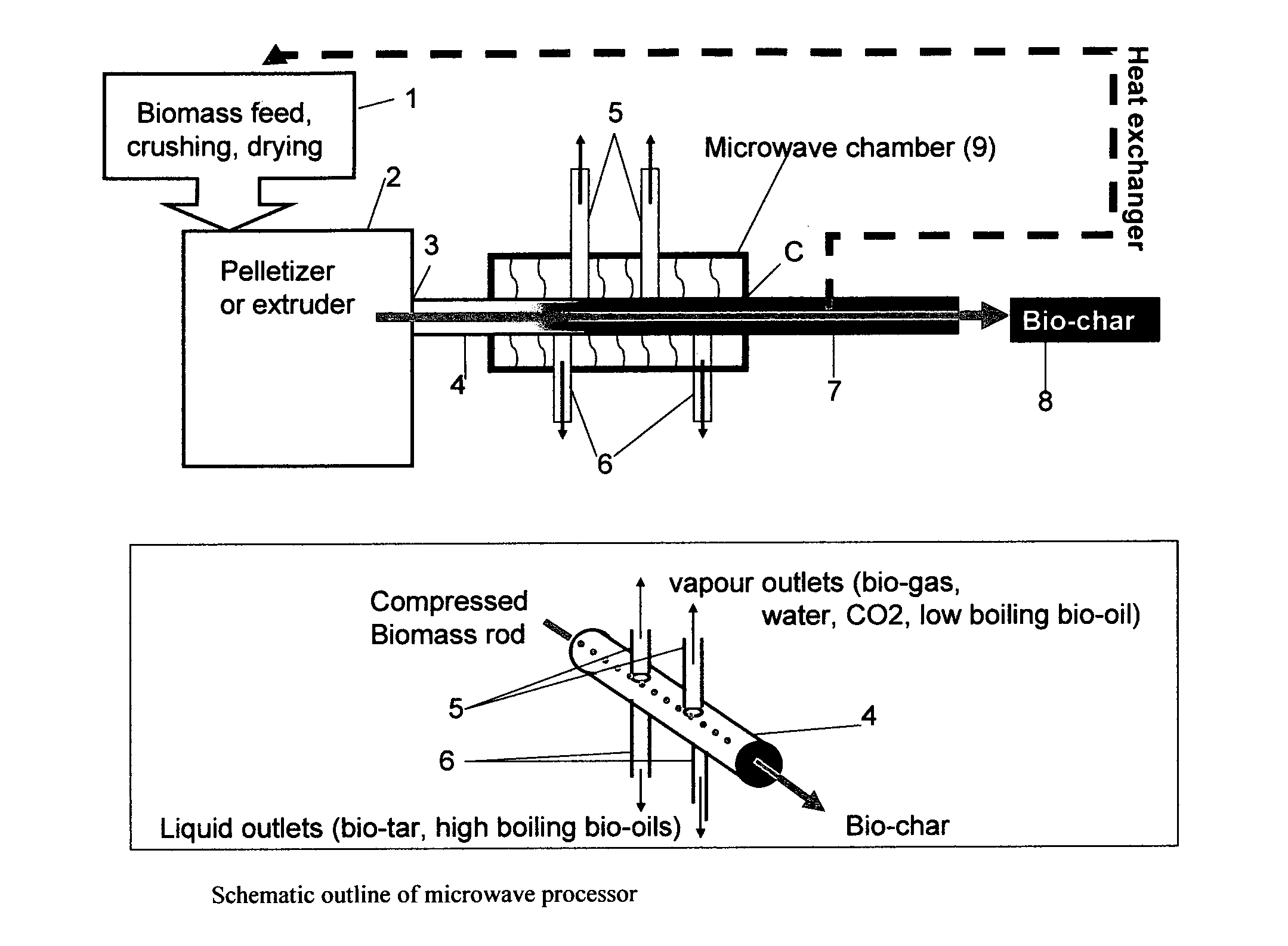
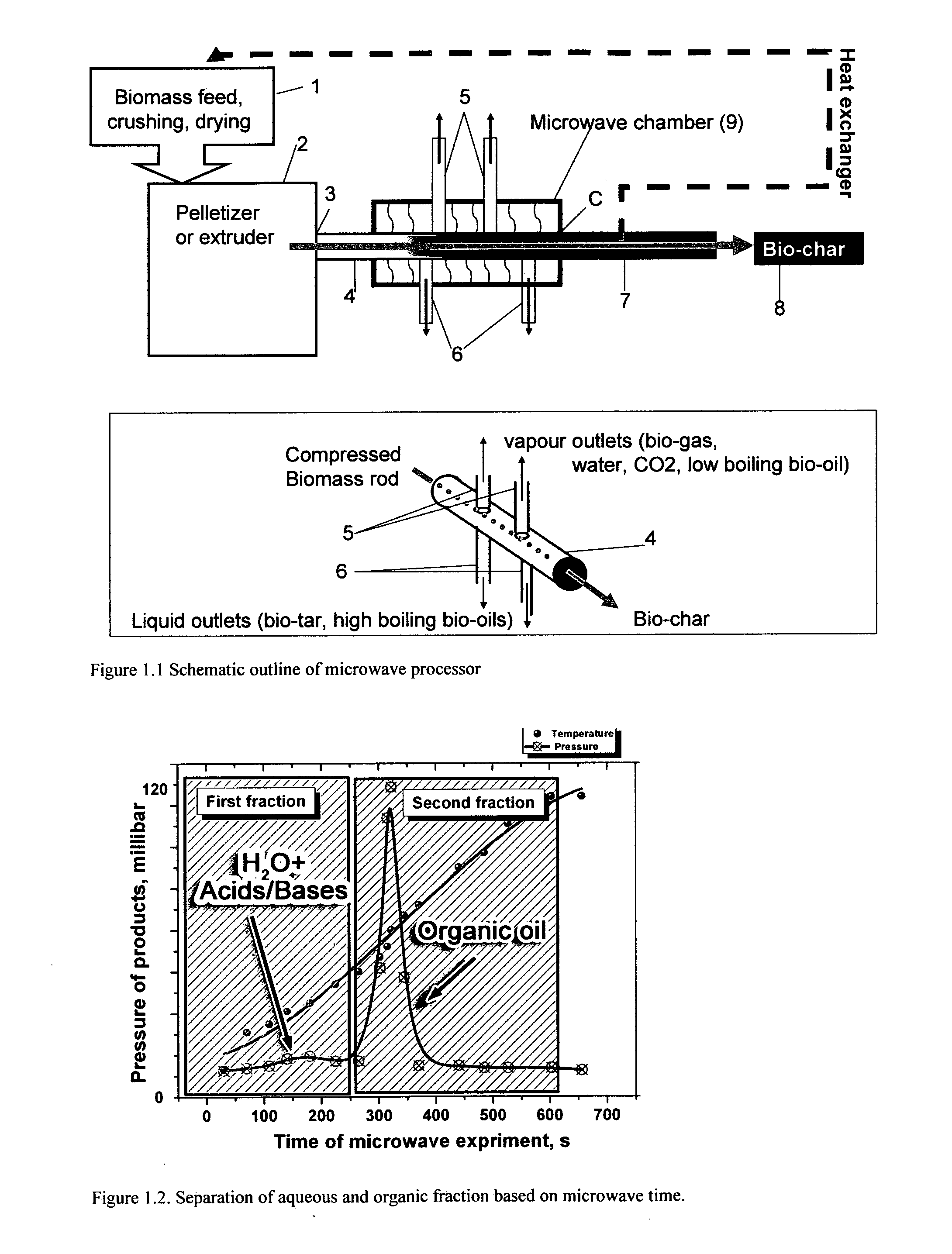
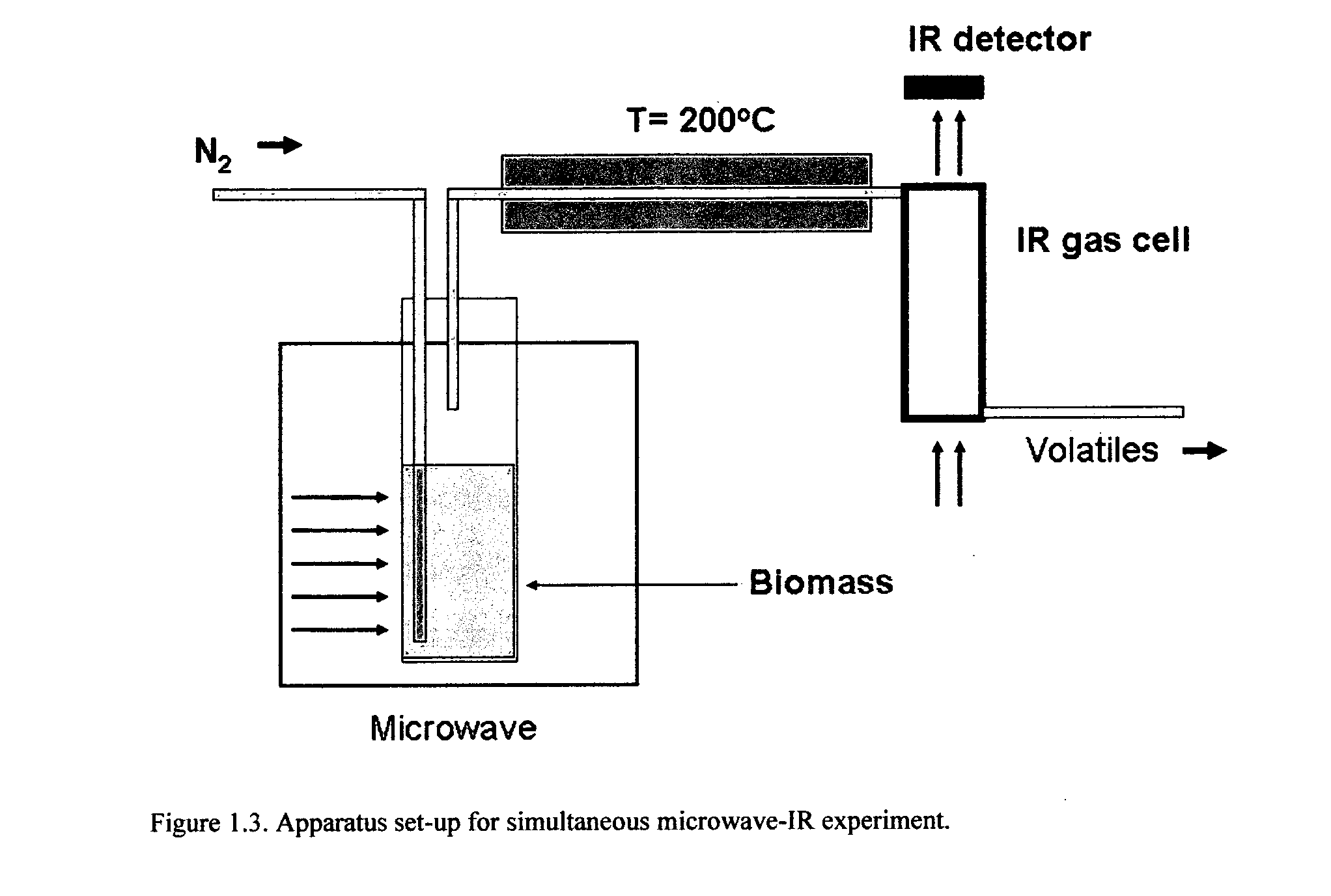
Microwave torrefaction of biomass
InactiveUS20110219679A1Increase flexibilityEasy to controlElectrical coke oven heatingBiofuelsMicrowaveTorrefaction
Owner:THE UNIV OF YORK
Process for microwave decomposition of hazardous matter
InactiveUS6187988B1Promote oxidationNitrogen compoundsLiquid separation by electricityActivated carbonHydrazine compound
This process occurs in the presence of activated carbon or its equivalent by decomposing adsorbed hazardous materials, such as hydrazine and microorganisms, on the carbon surface by radiofrequency energy in the microwave range at near ambient conditions of temperature and pressure. Further microwave oxidation to nonhazardous gases occurs in the presence of a microwaves enhanced oxidation catalyst.
Owner:CHA CHANG YUL
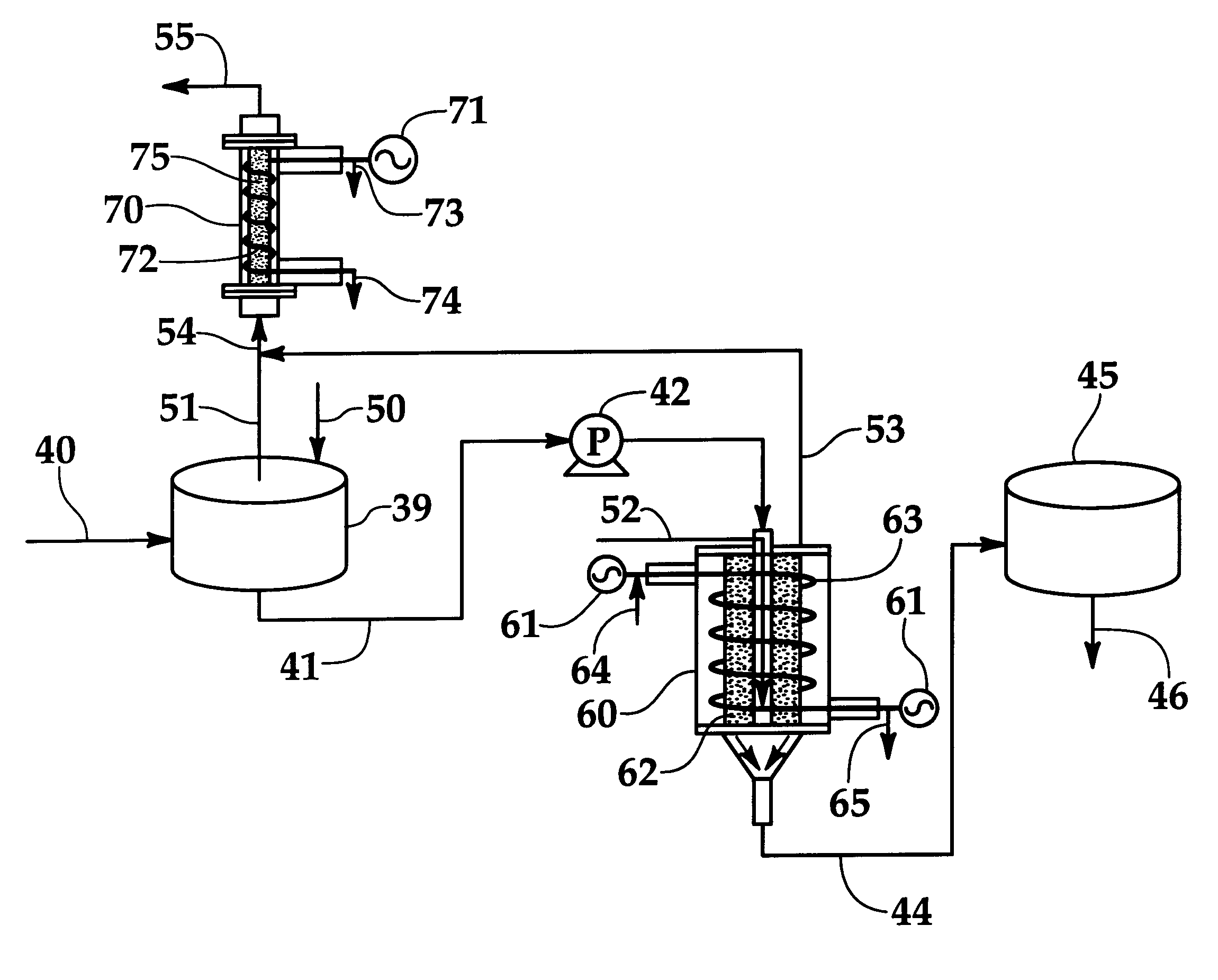
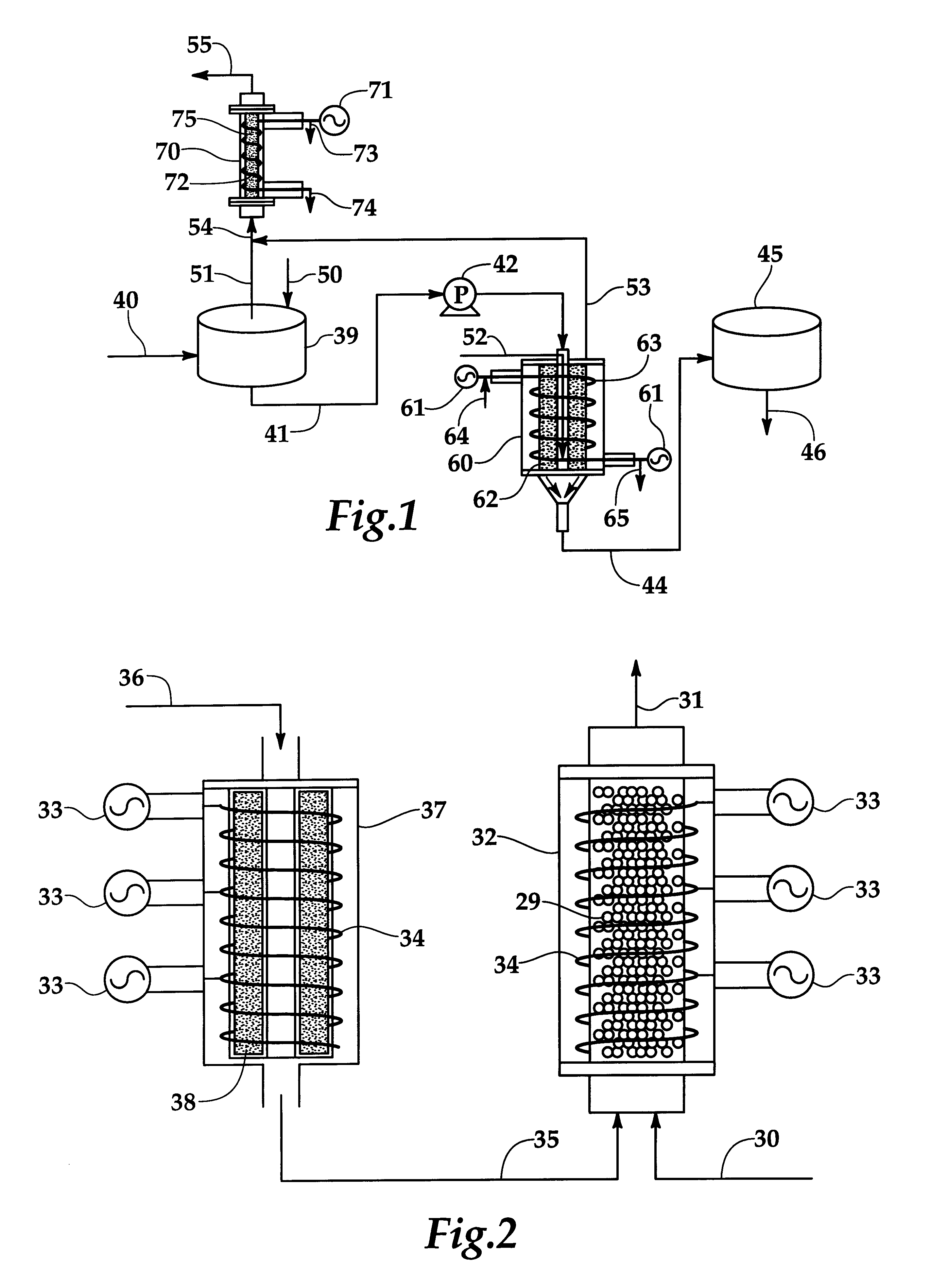
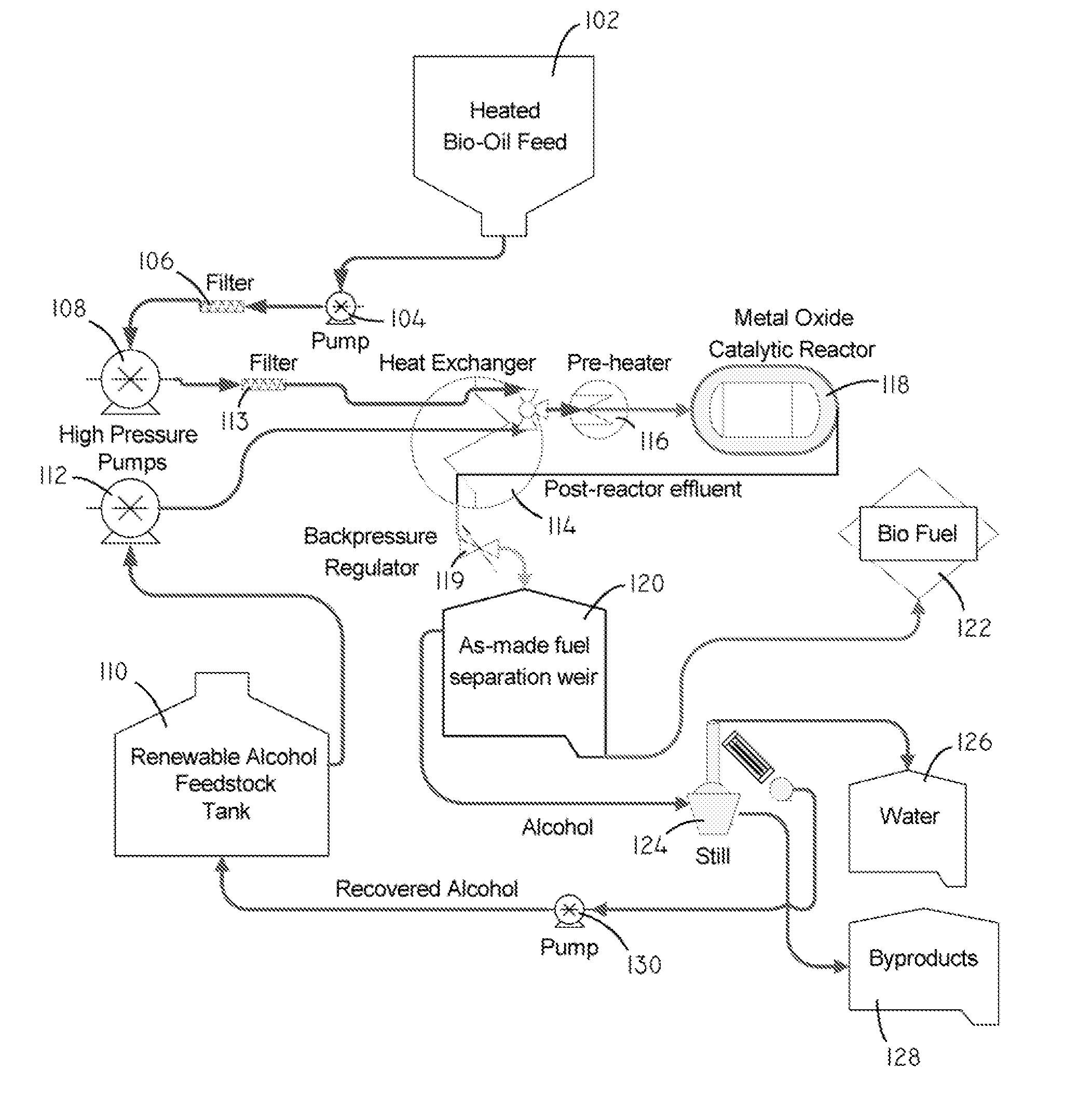
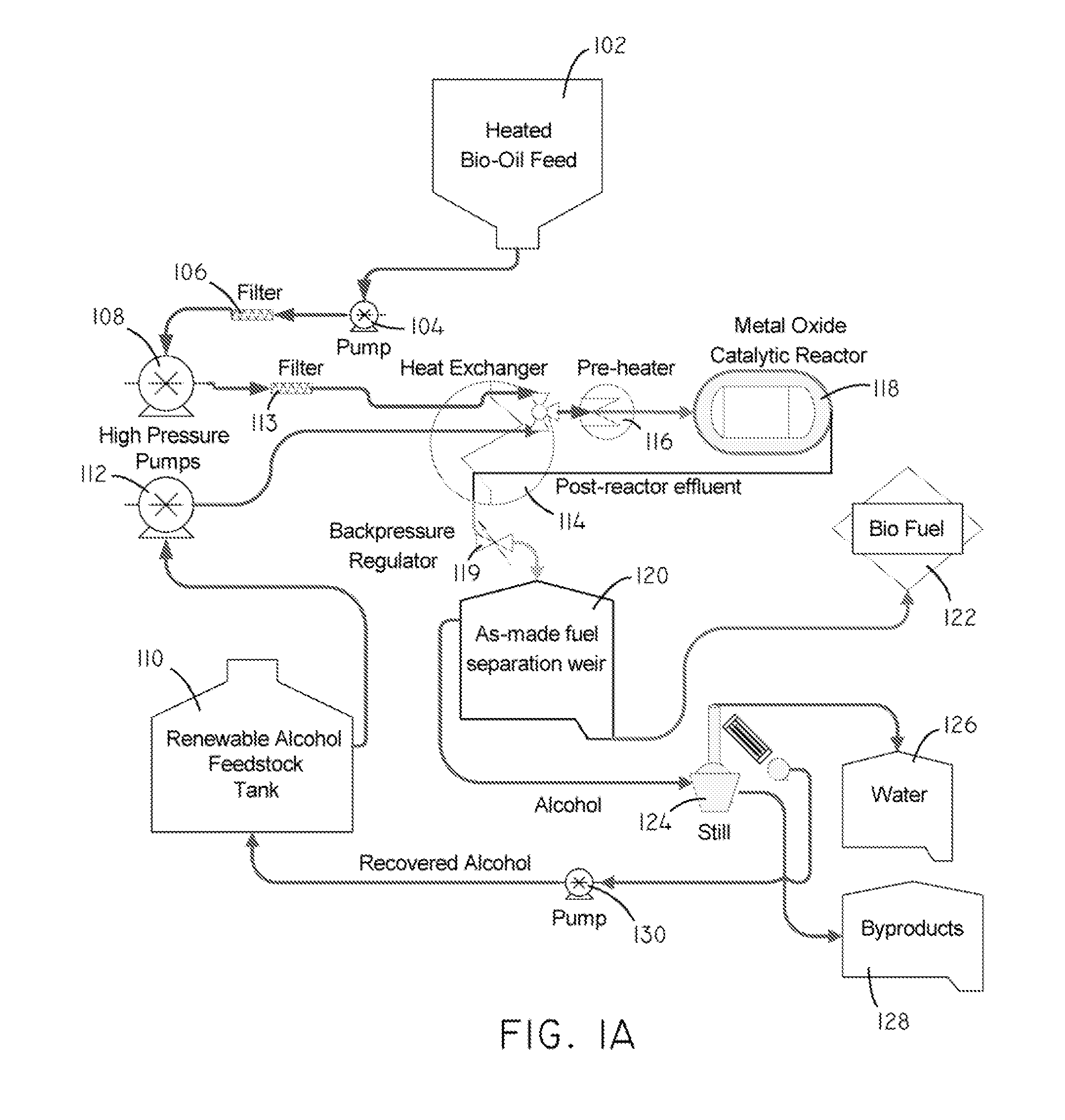
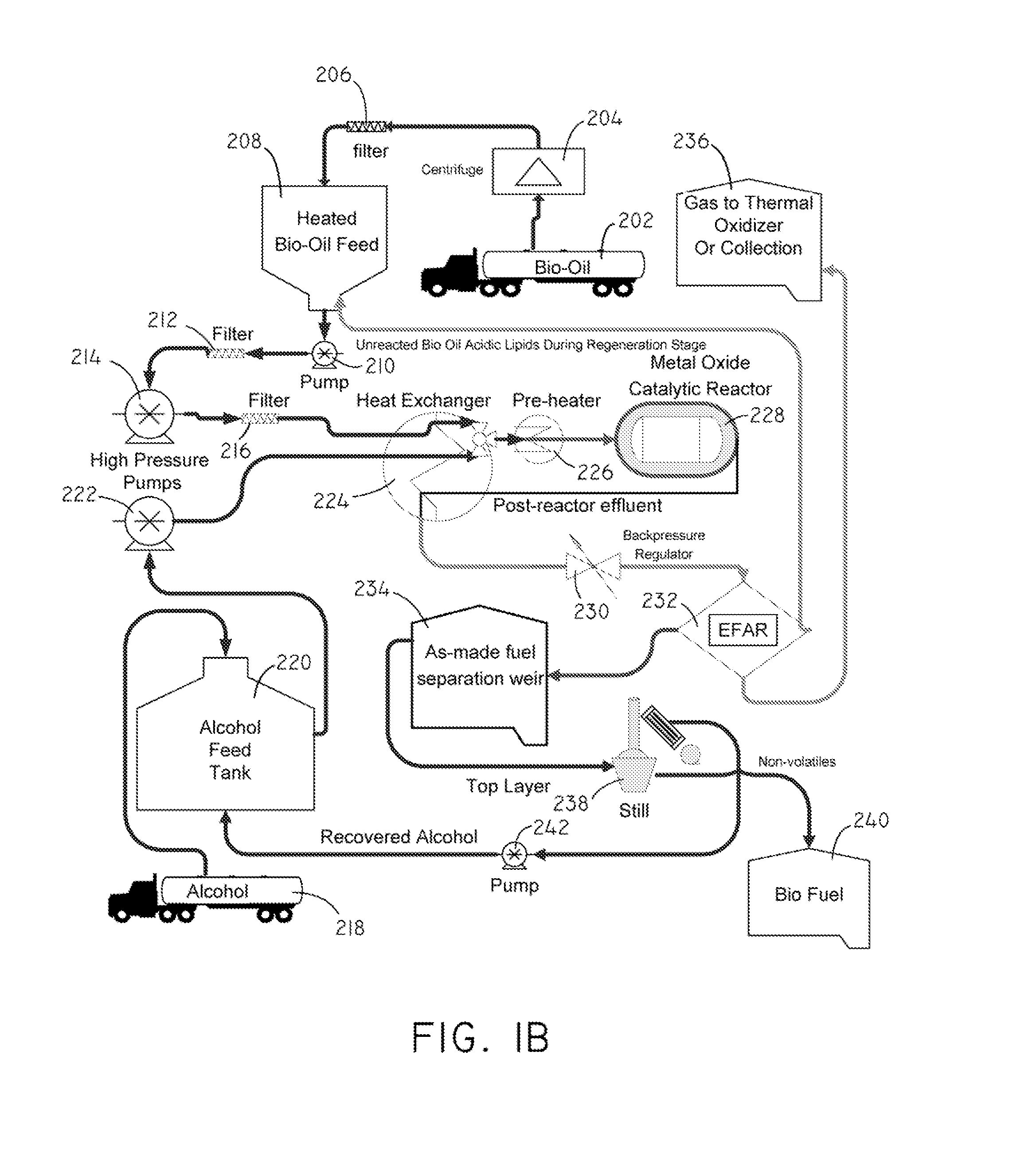
Systems and methods for producing fuels from biomass
The present invention relates to systems and methods for producing fuels. In an embodiment, the invention includes a method of producing a diesel-equivalent fuel, including pyrolyzing biomass to form a pyrolysis oil and contacting the pyrolysis oil and an alcohol with a metal oxide catalyst at a temperature of greater than about 60 degrees Celsius. In an embodiment, the invention includes a method of refining pyrolysis oil including contacting pyrolysis oil and an alcohol with a metal oxide catalyst at a temperature of greater than about 60 degrees Celsius. In an embodiment, the invention includes a system for processing biomass into fuel including a pyrolysis chamber defining an interior volume; a first heating element configured to heat the pyrolysis chamber; a refining chamber in selective fluid communication with the pyrolysis chamber, the refining chamber defining an interior volume, a metal oxide catalyst disposed within the interior volume; and a second heating element configured to heat the refining chamber. Other embodiments are also described herein.
Owner:SARTEC
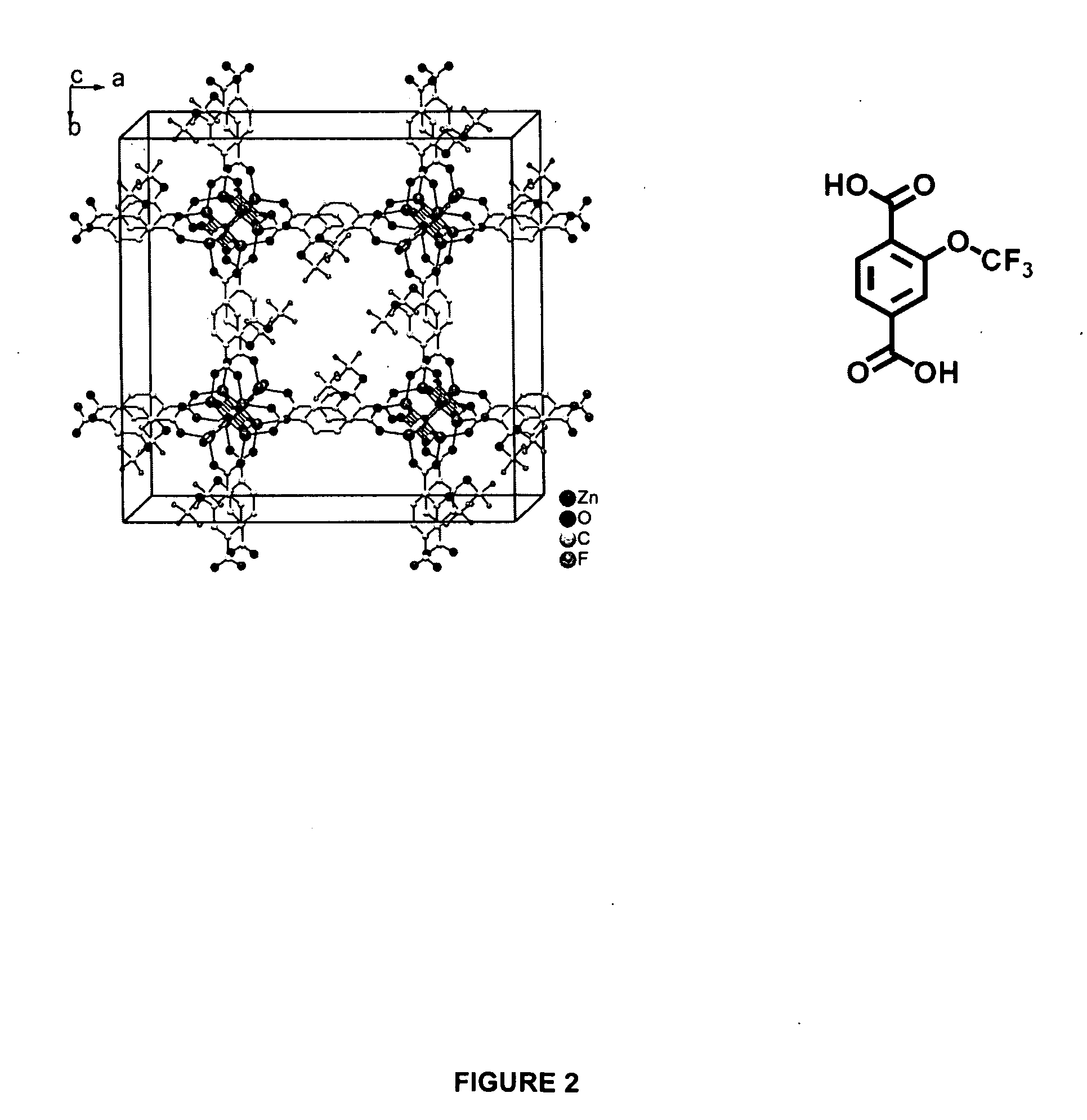
Water repellent metal-organic frameworks, process for making and uses regarding same
ActiveUS20100075123A1Improve stabilityImproved MOF stabilityGroup 8/9/10/18 element organic compoundsLayered productsWater vaporSorbent
Microwave assisted synthesis may be used to produce water-repellent metallic organic frameworks (MOFs) molecules. The water-repellent MOFs contain non-polar functional groups, such as a trifluoromethoxy group, which has a strong water repellent effect. The water-repellent MOF, when exposed to water vapor for one week does not result in a significant X-ray power pattern change. The water-repellent MOFs may be suitable as an adsorbent in many industrial applications, such as gas chromatography.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
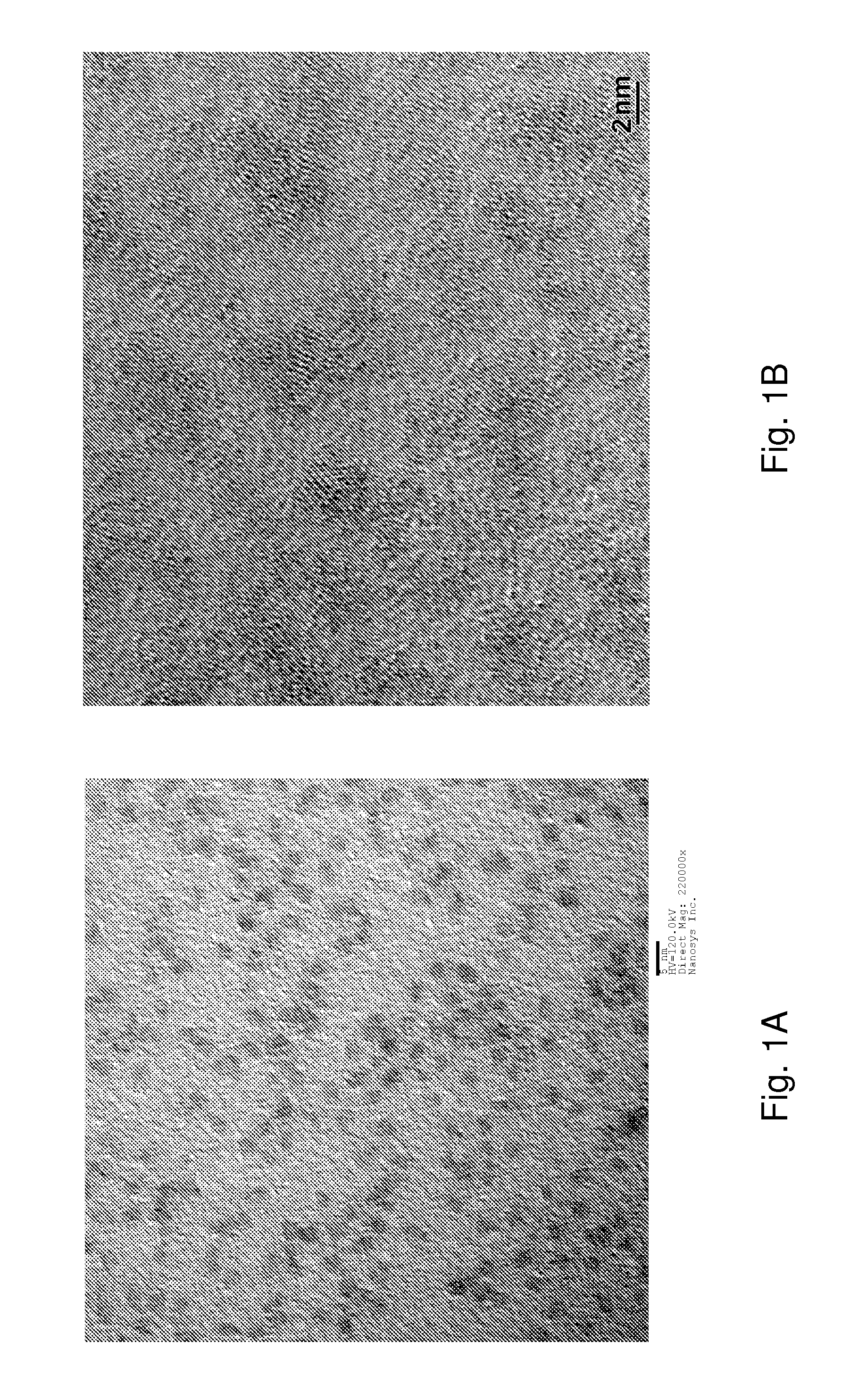
Highly Luminescent Nanostructures and Methods of Producing Same
ActiveUS20140001405A1High quantum yieldControl thicknessNickel organic compoundsNanoopticsLuminescence quantum yieldIndium
Highly luminescent nanostructures, particularly highly luminescent quantum dots, are provided. The nanostructures have high photoluminescence quantum yields and in certain embodiments emit light at particular wavelengths and have a narrow size distribution. The nanostructures can comprise ligands, including C5-C8 carboxylic acid ligands employed during shell formation and / or dicarboxylic or polycarboxylic acid ligands provided after synthesis. Processes for producing such highly luminescent nanostructures are also provided, including methods for enriching nanostructure cores with indium and techniques for shell synthesis.
Owner:NANOSYS INC
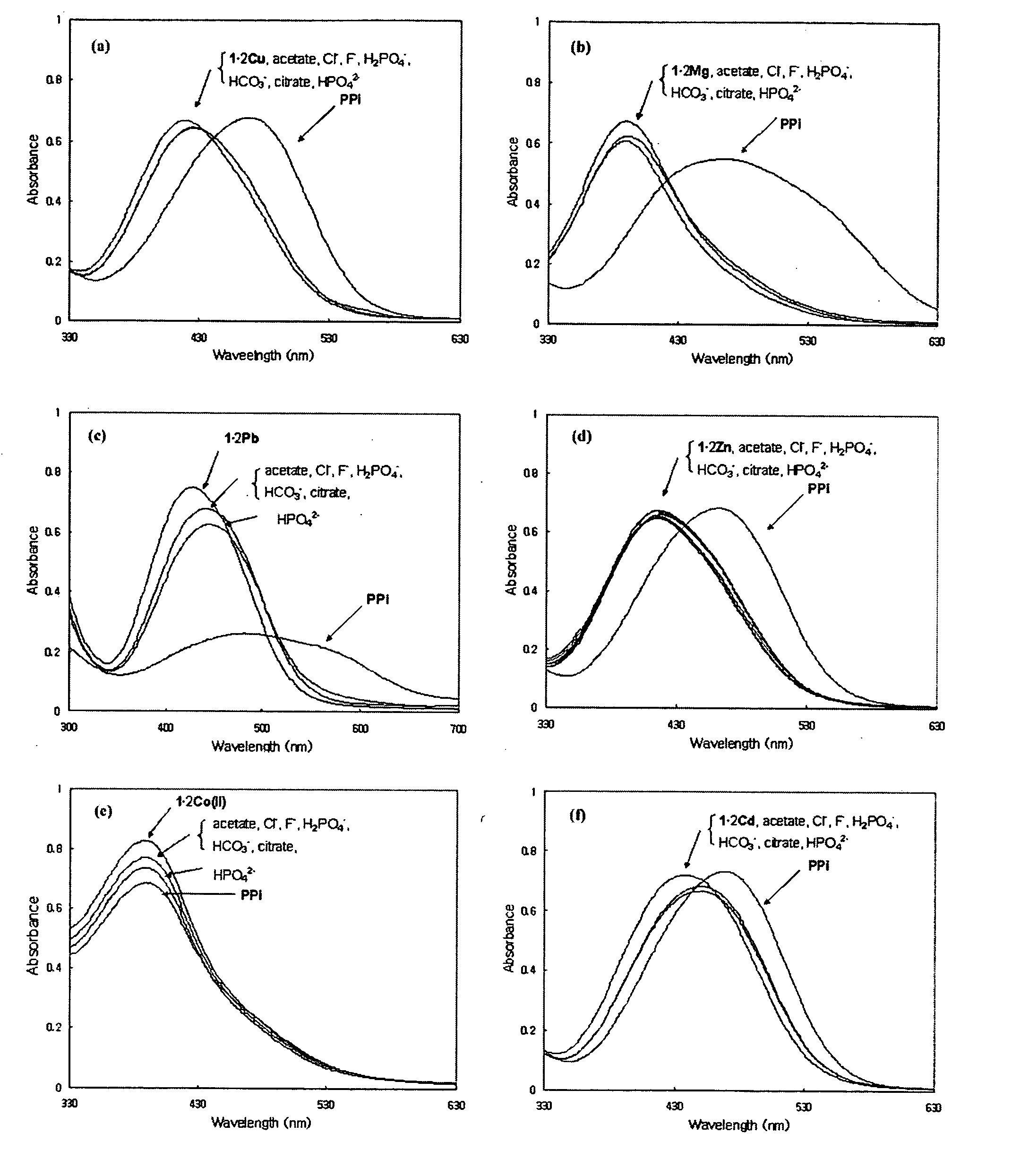
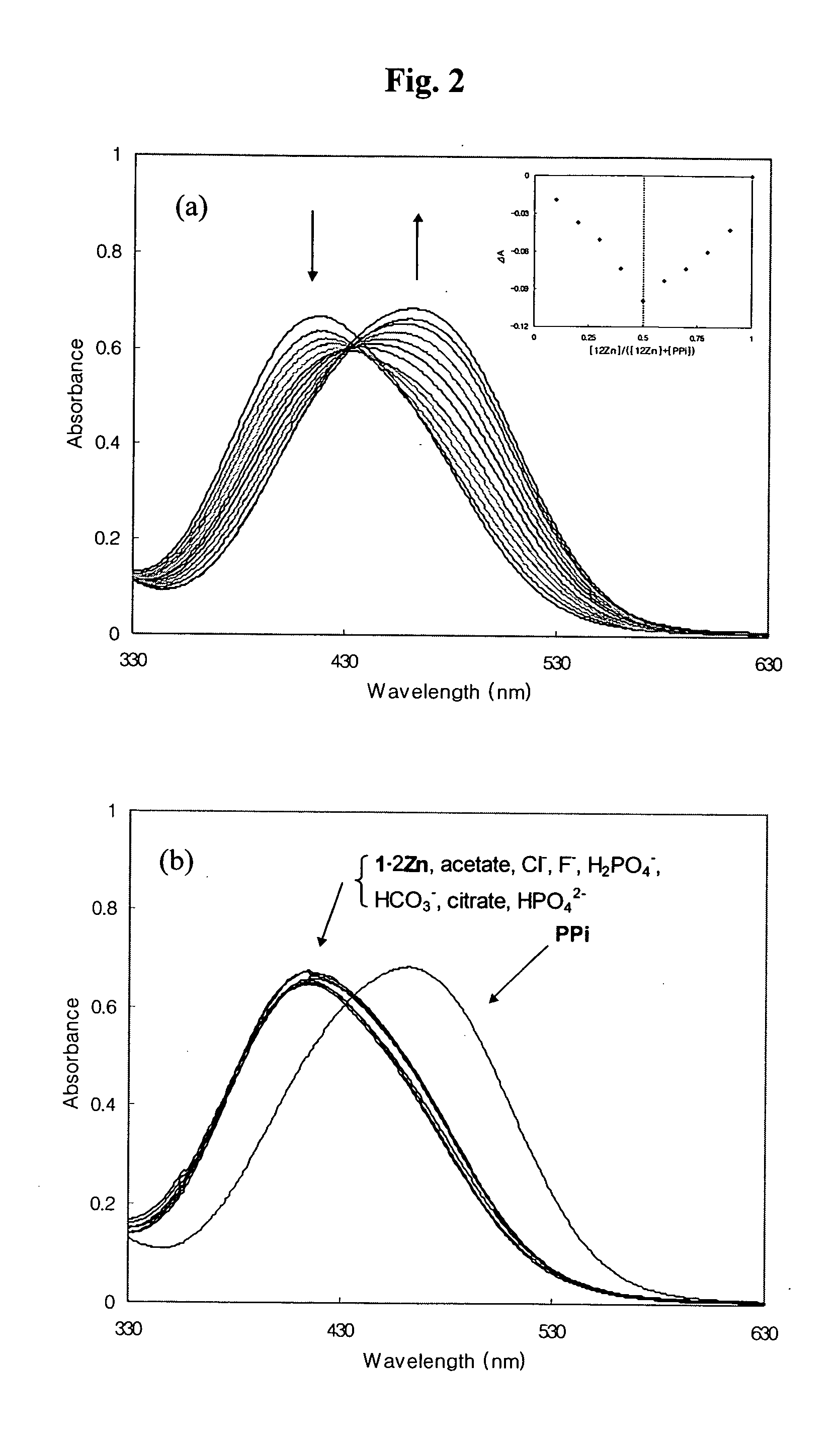
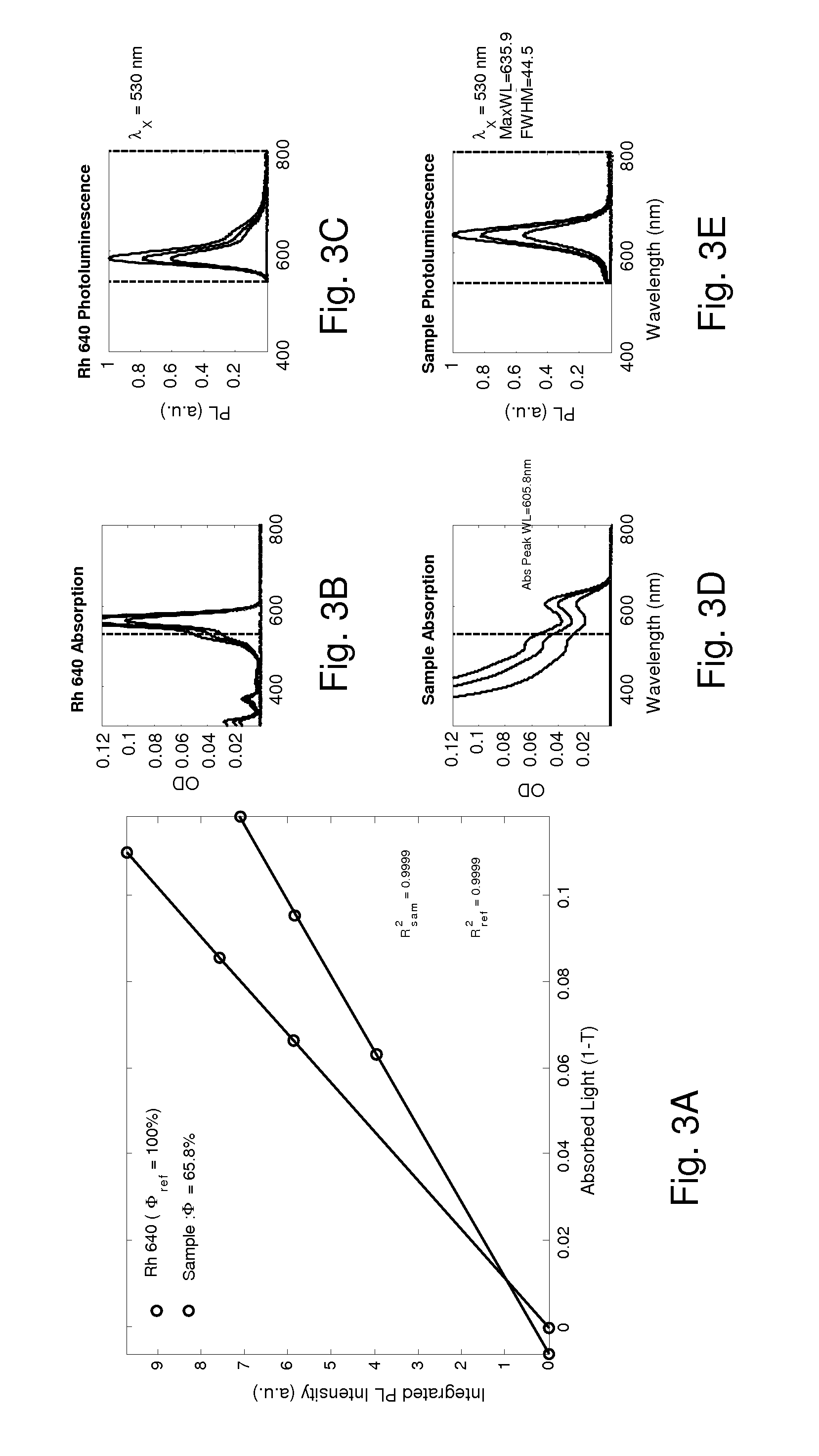
Dinuclear metal complex and pyrophosphate assay using the same
InactiveUS7279588B2Ultrasonic/sonic/infrasonic diagnosticsGroup 8/9/10/18 element organic compoundsElectronic densityPyrophosphate
A novel coordination complex formed by dinuclear metal complexation is provided. The complex is a dinuclear metal complex of a compound, wherein the compound comprises a conjugation ring system substituted with: a) an electron donating group selected from —OH, —SH and —NH2; b) an indicating group selected from a chromogenic group, a fluorescent group and an electrochemical group; and c) two binding auxiliary groups, in combination with the electron donating group each of which being coordinated with the metal to provide an anion bonding site, wherein as the complex binds to a anion, the coordination of the electron donating group with the metal is weakened and electron donation of the electron donating group to the conjugation ring system is reinforced such that the reinforced electron donation by the electron donating group is transferred through the conjugation ring system to the indicating group to produce an indicating signal concomitant with the change of its electronic density. The coordination complex shows high sensitivity and high selectivity for pyrophosphate over other anions in an aqueous solvent over a wide pH range. Therefore, the complex is useful for pyrophosphate assay as a pyrophosphate sensor.
Owner:SEOUL NAT UNIV FOUND
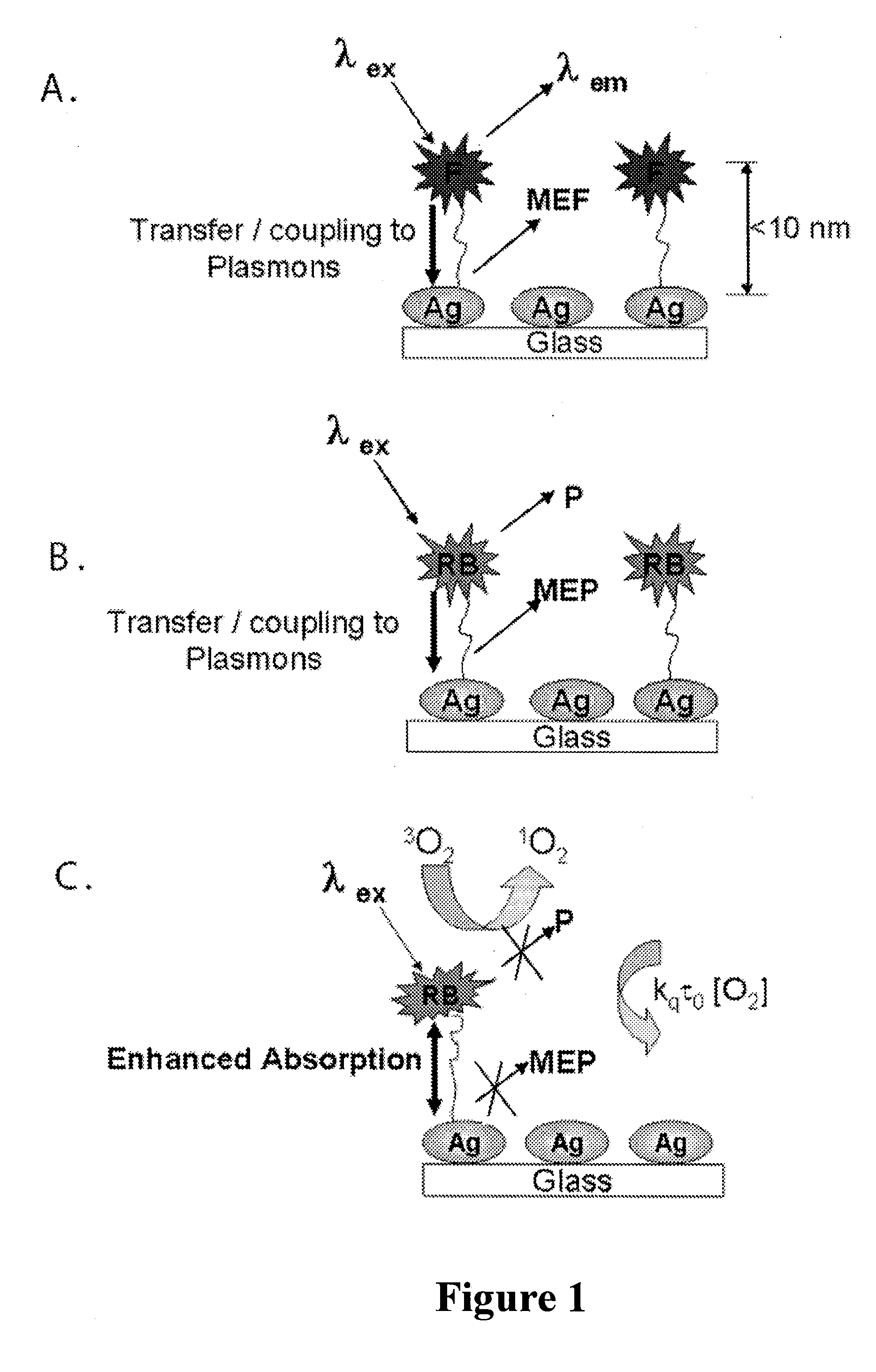
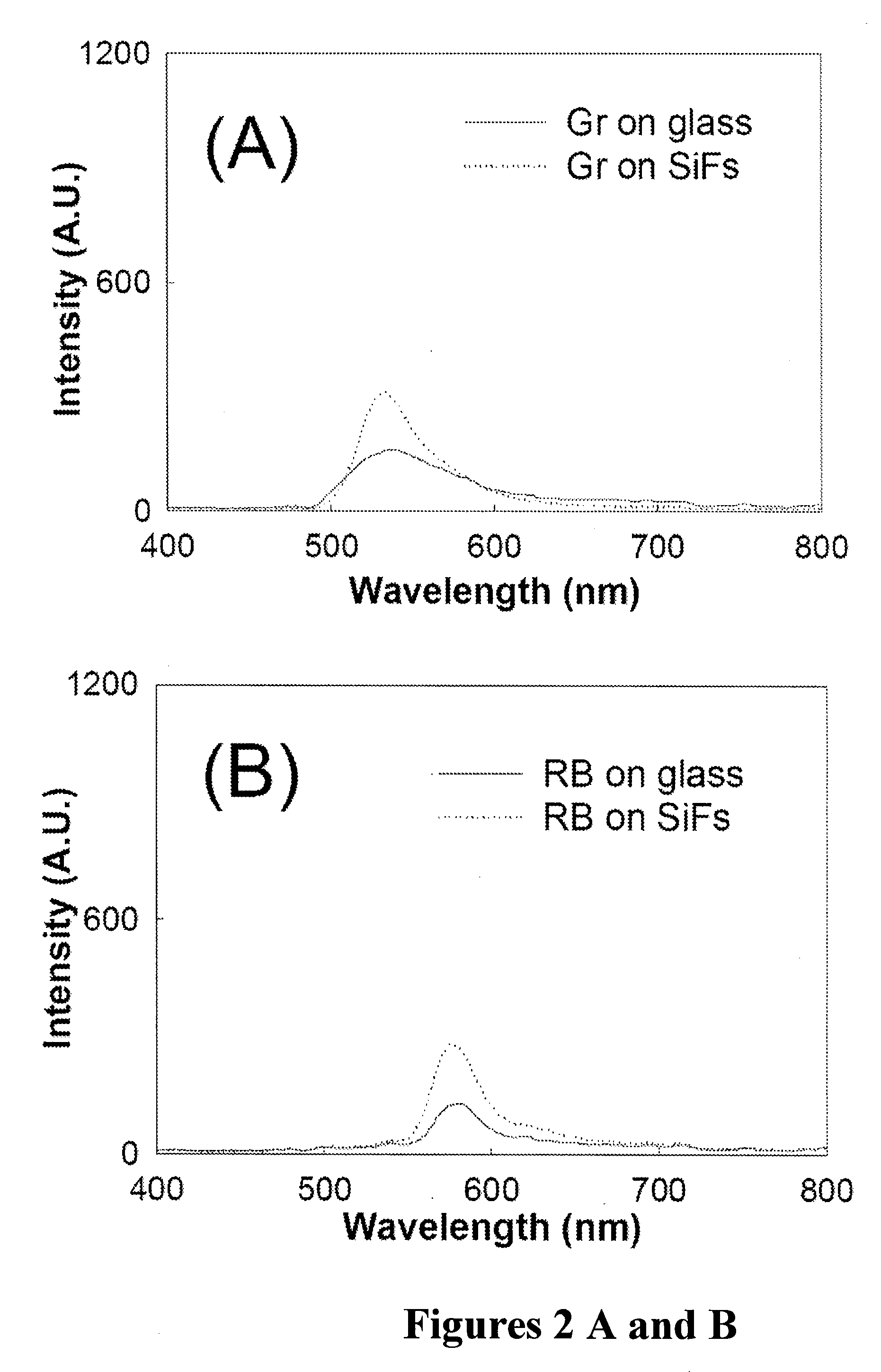
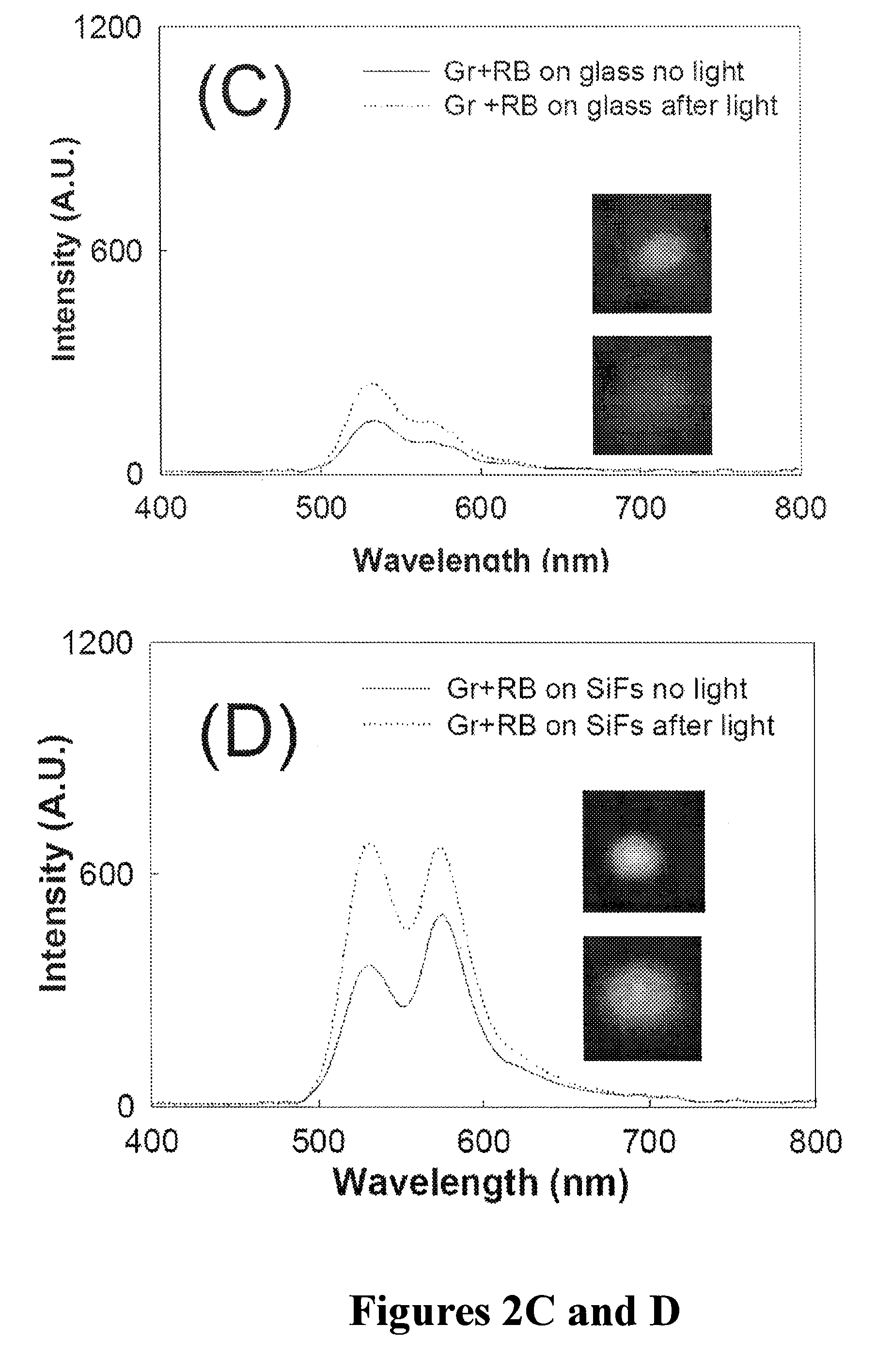
Plasmonic engineering of singlet oxygen and/or superoxide generation
ActiveUS20080215122A1Increase triplet yieldIncreased oxygen generationBiocideGroup 8/9/10/18 element organic compoundsEnergy absorptionSuperoxide
The present invention provides for a method to increase the triplet yield of a photosensitizer by the coupling to metal surface plasmons which leads to increased singlet oxygen generation by electric field enhancement or enhanced energy absorption of the photosensitizer. The extent of singlet oxygen enhancement can be tuned for applications in singlet oxygen based clinical therapy by modifying plasmon coupling parameters, such as metallic nanoparticle size and shape, photosensitizer / metallic nanoparticle distance, and the excitation wavelength of the coupling photosensitizer.
Owner:UNIV OF MARYLAND BALTIMORE COUNTY
Microwave induced functionalization of single wall carbon nanotubes and composites prepared therefrom
InactiveUS20060210466A1Rapid funtionalizationHigh purityMaterial nanotechnologyGroup 1/11 element organic compoundsCarbon nanotubeMicrowave electromagnetic radiation
The invention is directed to a method of forming, producing or manufacturing functionalized nanomaterials, and, specifically, soluble functionalized nanomaterials. The presently described invention also relates to nanomaterial-based composites consisting of a target material, which can include ceramic, polymer, or metallic matrices incorporated into or grown on nanomaterials, as well as a method or synthesis technique for the formation, production, or manufacture of nanomaterial-based composites through microwave-induced reaction.
Owner:NEW JERSEY INSTITUTE OF TECHNOLOGY
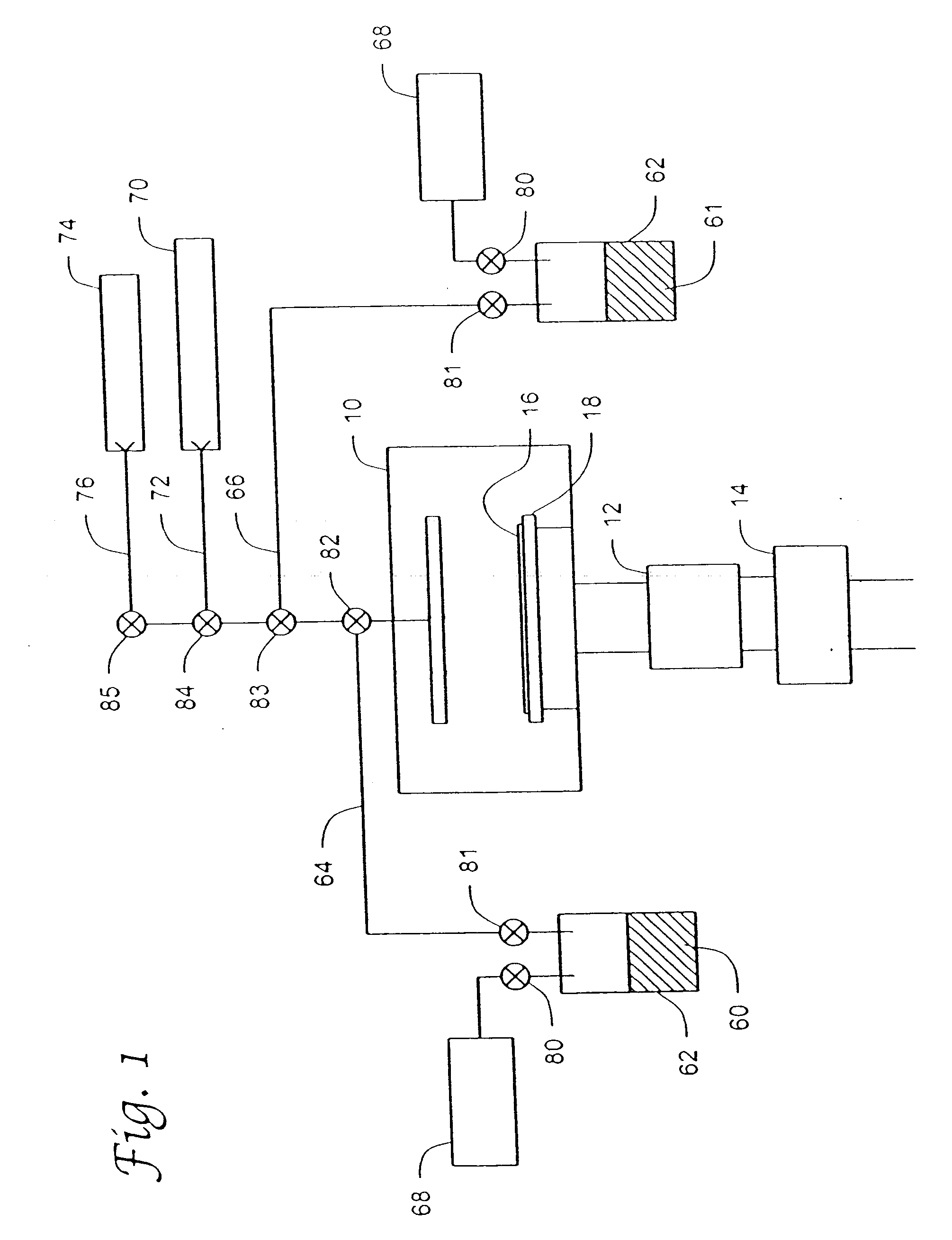
Reaction and temperature control for high power microwave-assisted chemistry techniques
InactiveUS6917023B2Organic chemistry methodsDielectric heating circuitsMicrowave cavityTemperature control
A method is disclosed for carrying out microwave assisted chemical reactions. The method includes the steps of placing reactants in a microwave-transparent vessel, placing the vessel and its contents into a microwave cavity, applying microwave radiation within the cavity and to the vessel and its contents while concurrently externally cooling the vessel conductively.
Owner:CEM CORP
Compositions and Formulations for the Treatment of Halitosis
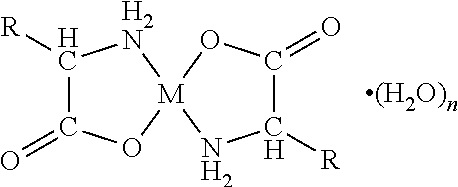
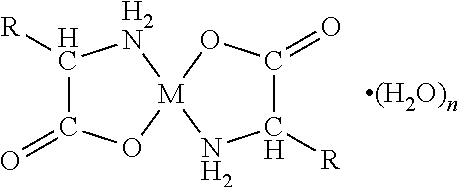
An orally disintegrating composition comprising a chelate comprising a metal ion and a biologically acceptable amino acid and having the general formulawherein M is the metal ion Zn2+ and R is H in the biologically acceptable amino acid glycine, use of the composition for the treatment of halitosis, and a chewing gum for the treatment of halitosis comprising a chelate comprising a metal ion and a biologically acceptable amino acid and having said general formula, which chewing gum is based on a carrier composition comprising a gum base, sorbitol, xylitol, one or more plasticizer(s), and one or more anticaking agent(s).
Owner:EJP PHARMA APS
Fluorinated dye stabilizers in fluorinated dielectric solvent
InactiveUS7141688B2Improve quenching efficiencyImprove solubilityGroup 1/11 element organic compoundsReactive dyesSolubilitySolvent
The present invention is directed to novel fluorinated dye stabilizers having both high quenching efficiency and solubility in halogenated solvents. These dye stabilizers have shown a significantly effect on improving the dye fastness in hostile photooxidation conditions.
Owner:E INK CALIFORNIA
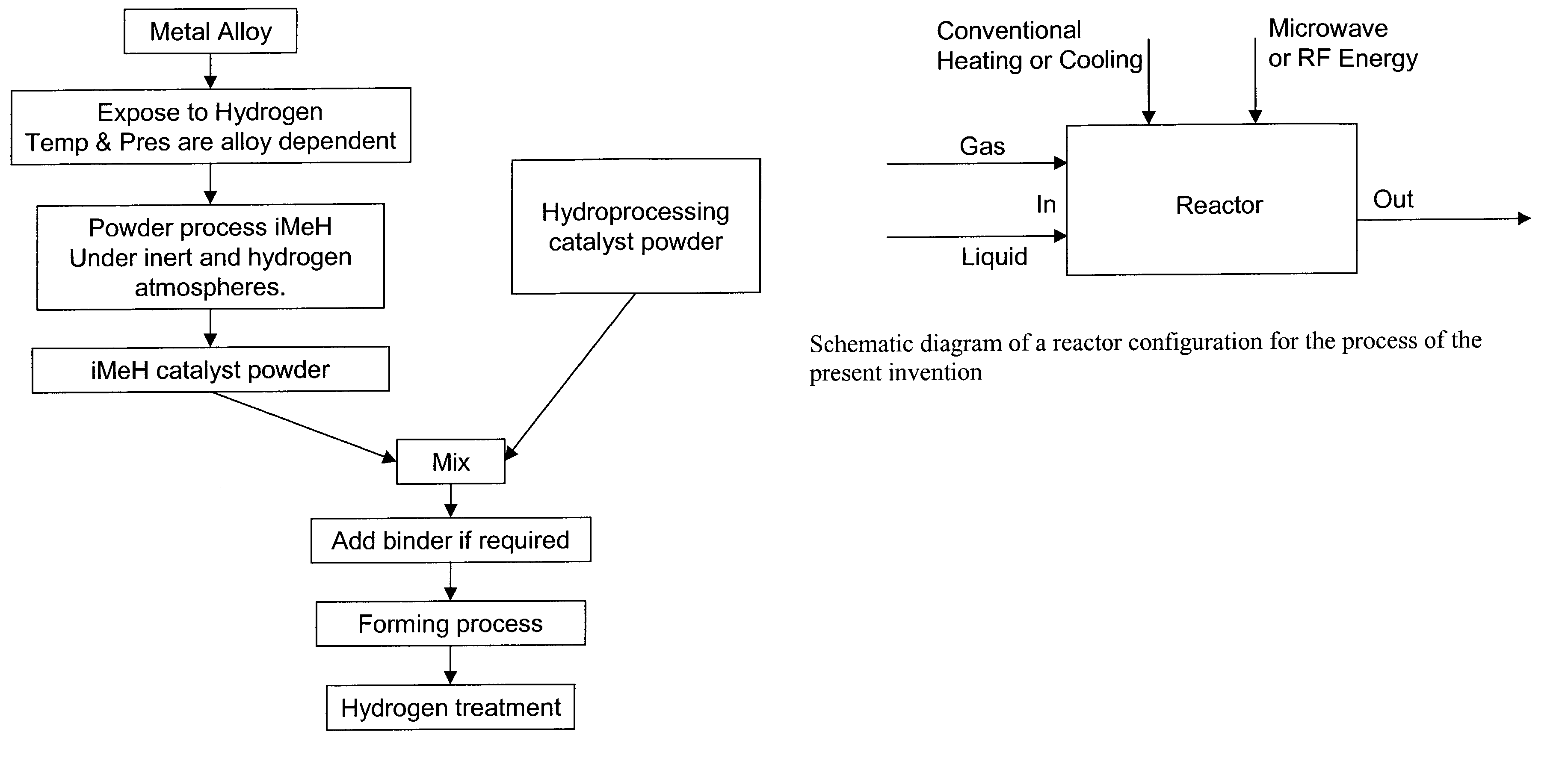
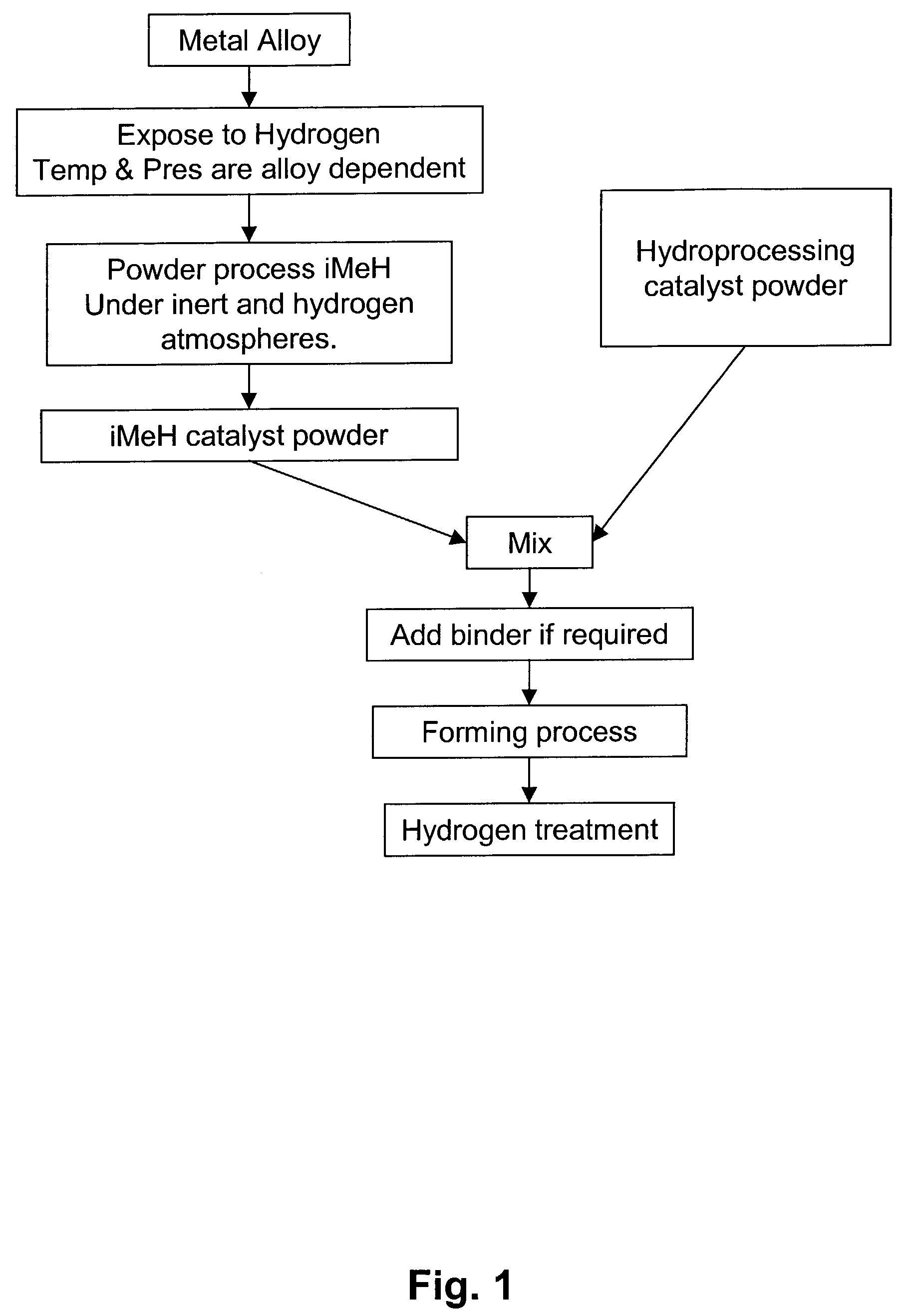
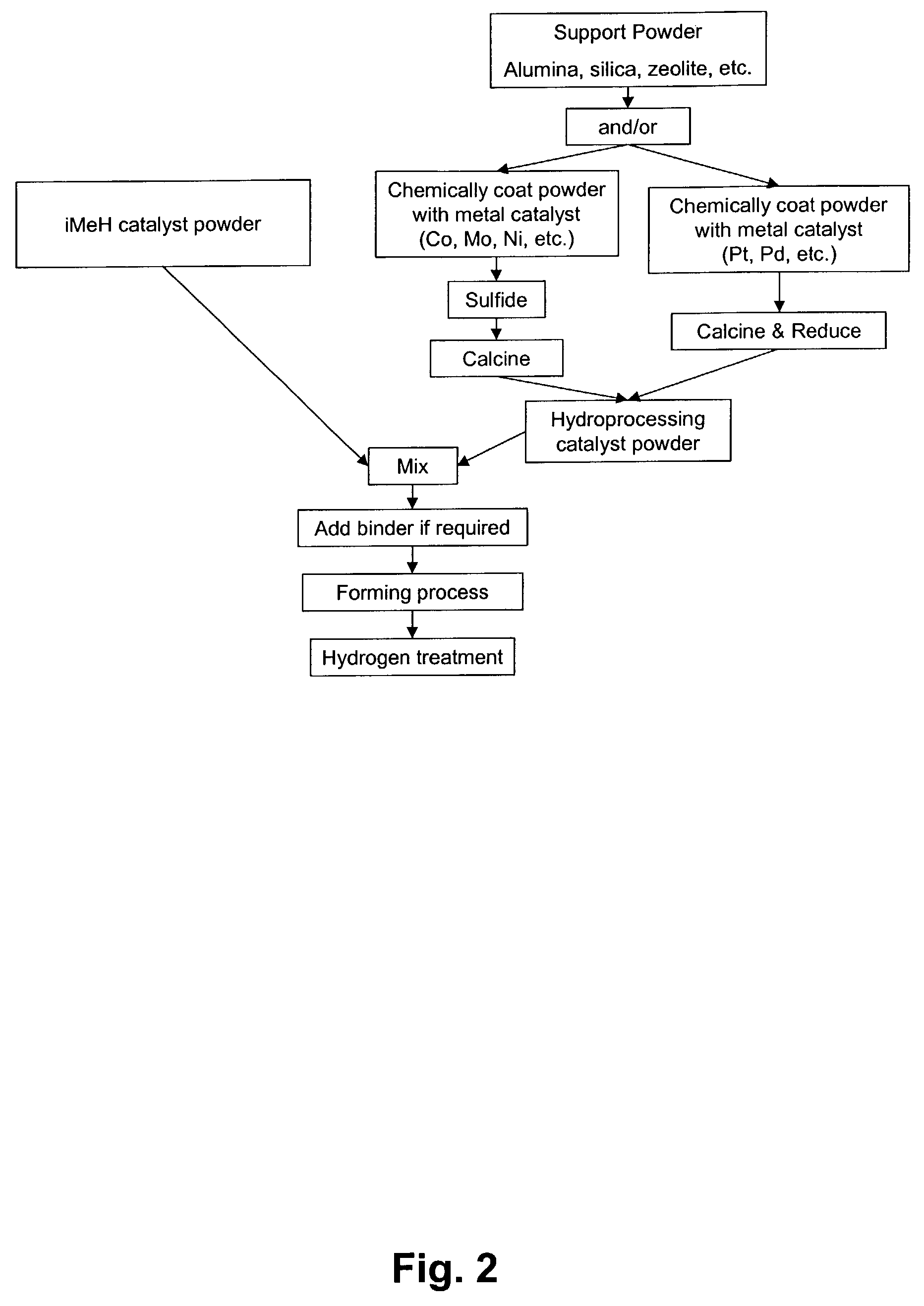
Catalytic process for the treatment of organic compounds
InactiveUS7387712B2Group 1/11 element organic compoundsCatalytic crackingSimple Organic CompoundsPtru catalyst
A process for the catalytic reaction of organic compounds, in which the organic compounds are contacted with a catalyst comprising an interstitial metal hydride, having a reaction surface, to produce a catalyst-organic compound mixture, energy is applied, monatomic hydrogen is produced at the reaction surface of the interstitial metal hydride, and the organic compounds are reacted with the monatomic hydrogen. Reactions accomplished by this process include petroleum hydrocracking and hydrotreating processes. The method's performance can be further enhanced using radio frequency (RF) or microwave energy.
Owner:CARNEGIE MELLON UNIV
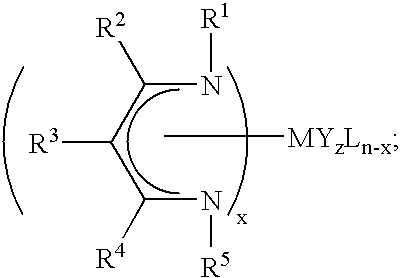
Beta-diketiminate ligand sources and metal-containing compounds thereof, and systems and methods including same
InactiveUS20060292303A1Increase vapor pressureLow melting pointSemiconductor/solid-state device manufacturingCobalt organic compoundsGas phaseΒ diketiminate
The present invention provides metal-containing compounds that include at least one β-diketiminate ligand, and methods of making and using the same. In certain embodiments, the metal-containing compounds include at least one β-diketiminate ligand with at least one fluorine-containing organic group as a substituent. In other certain embodiments, the metal-containing compounds include at least one β-diketiminate ligand with at least one aliphatic group as a substituent selected to have greater degrees of freedom than the corresponding substituent in the β-diketiminate ligands of certain metal-containing compounds known in the art. The compounds can be used to deposit metal-containing layers using vapor deposition methods. Vapor deposition systems including the compounds are also provided. Sources for β-diketiminate ligands are also provided.
Owner:MICRON TECH INC
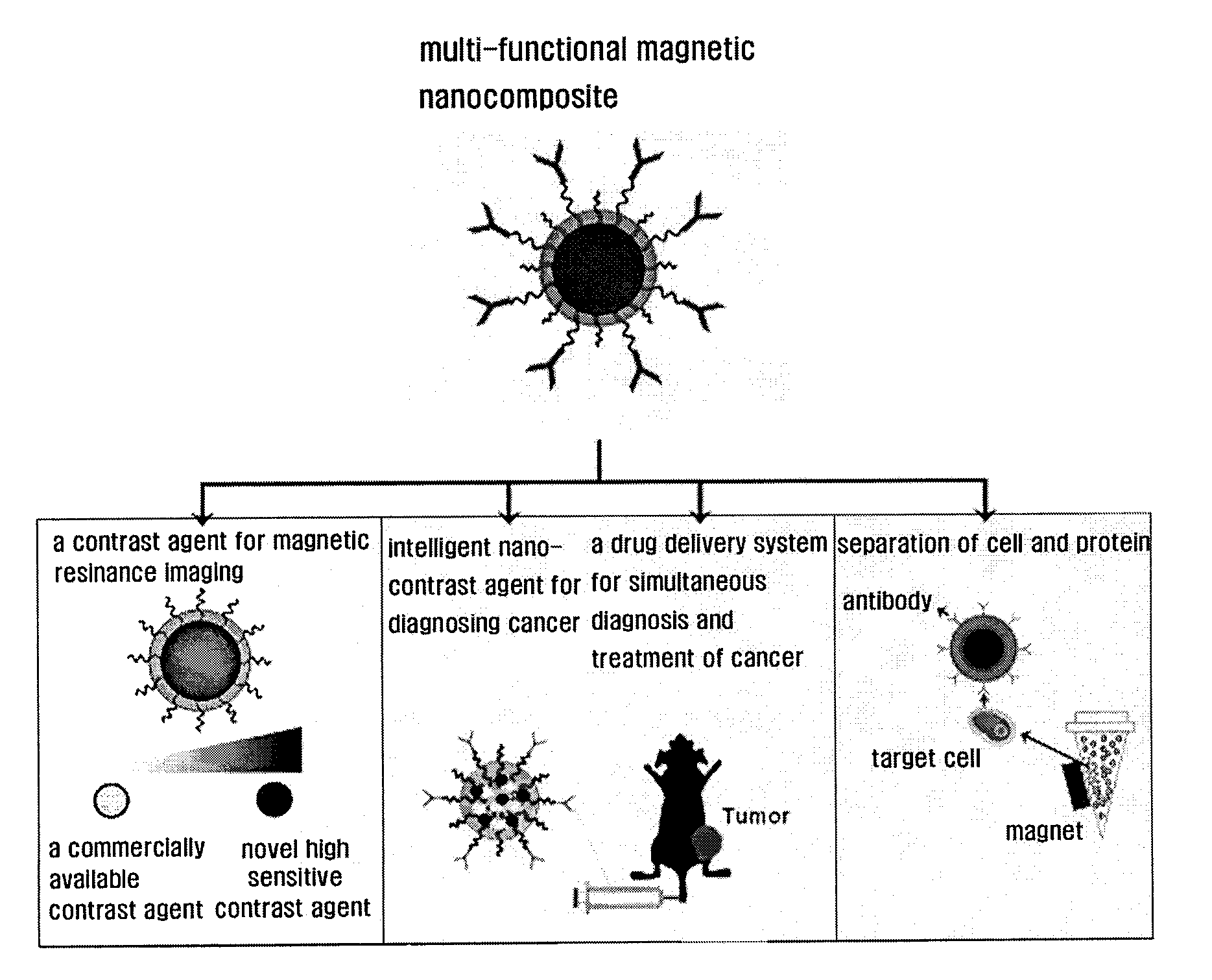
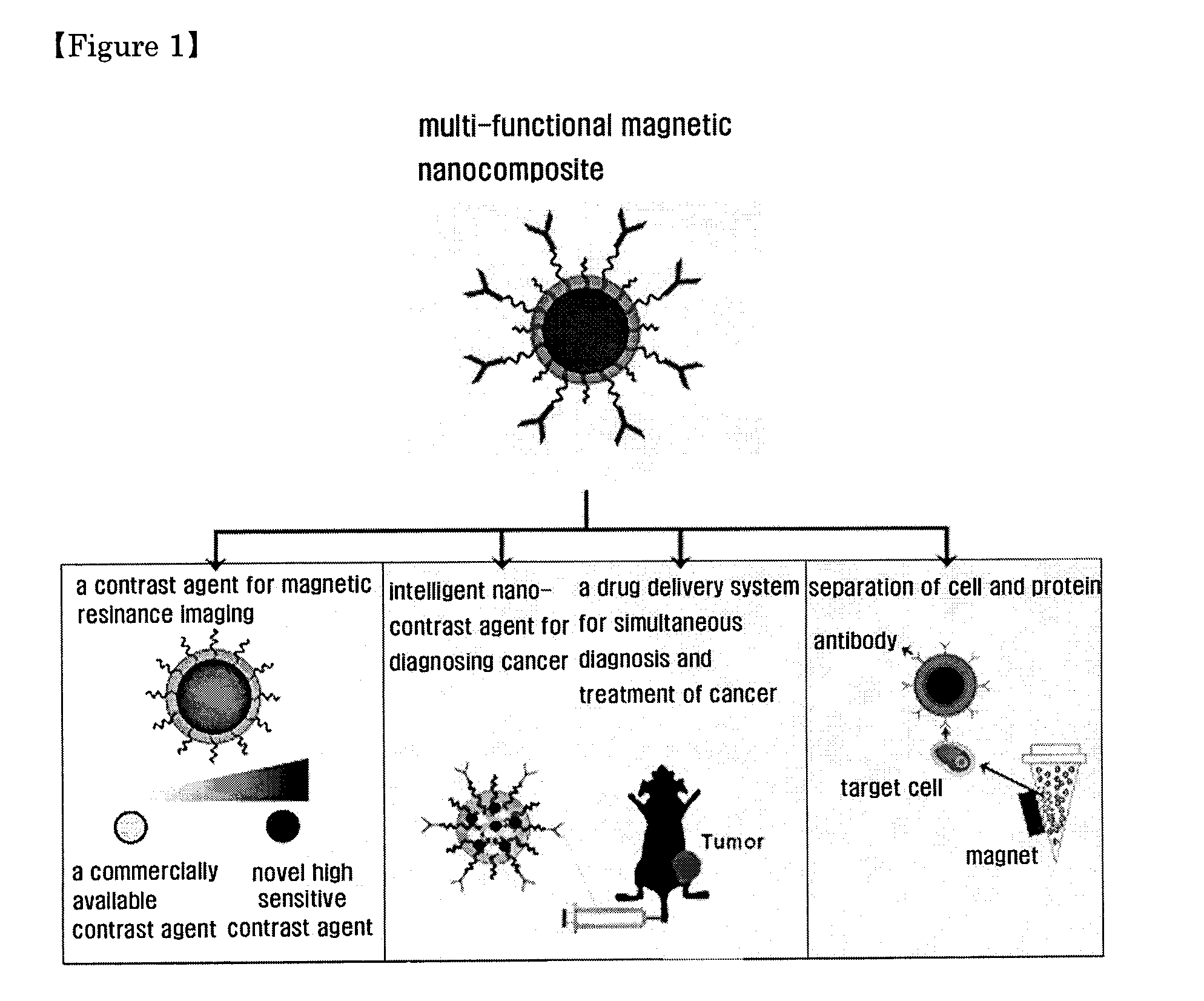
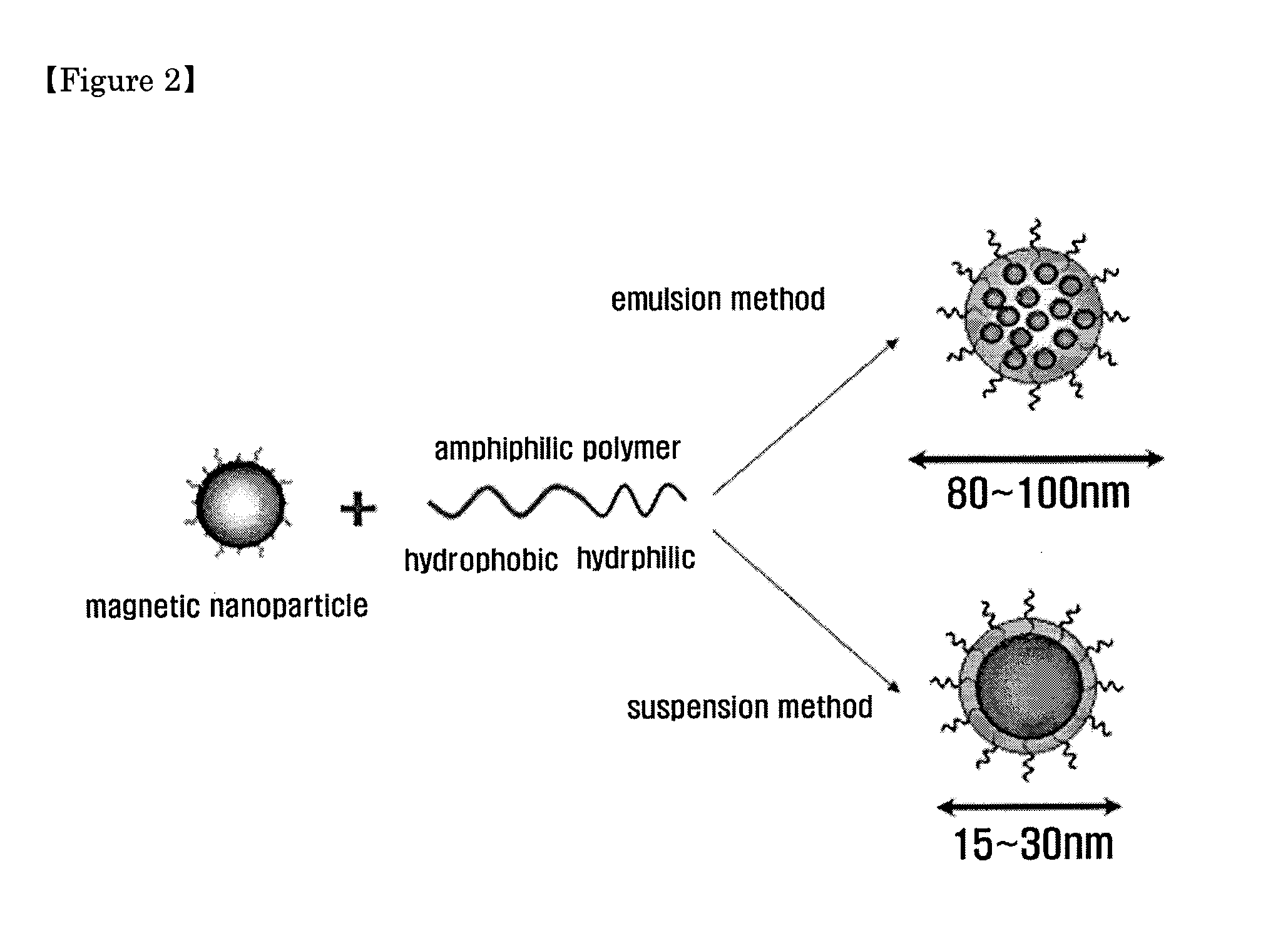
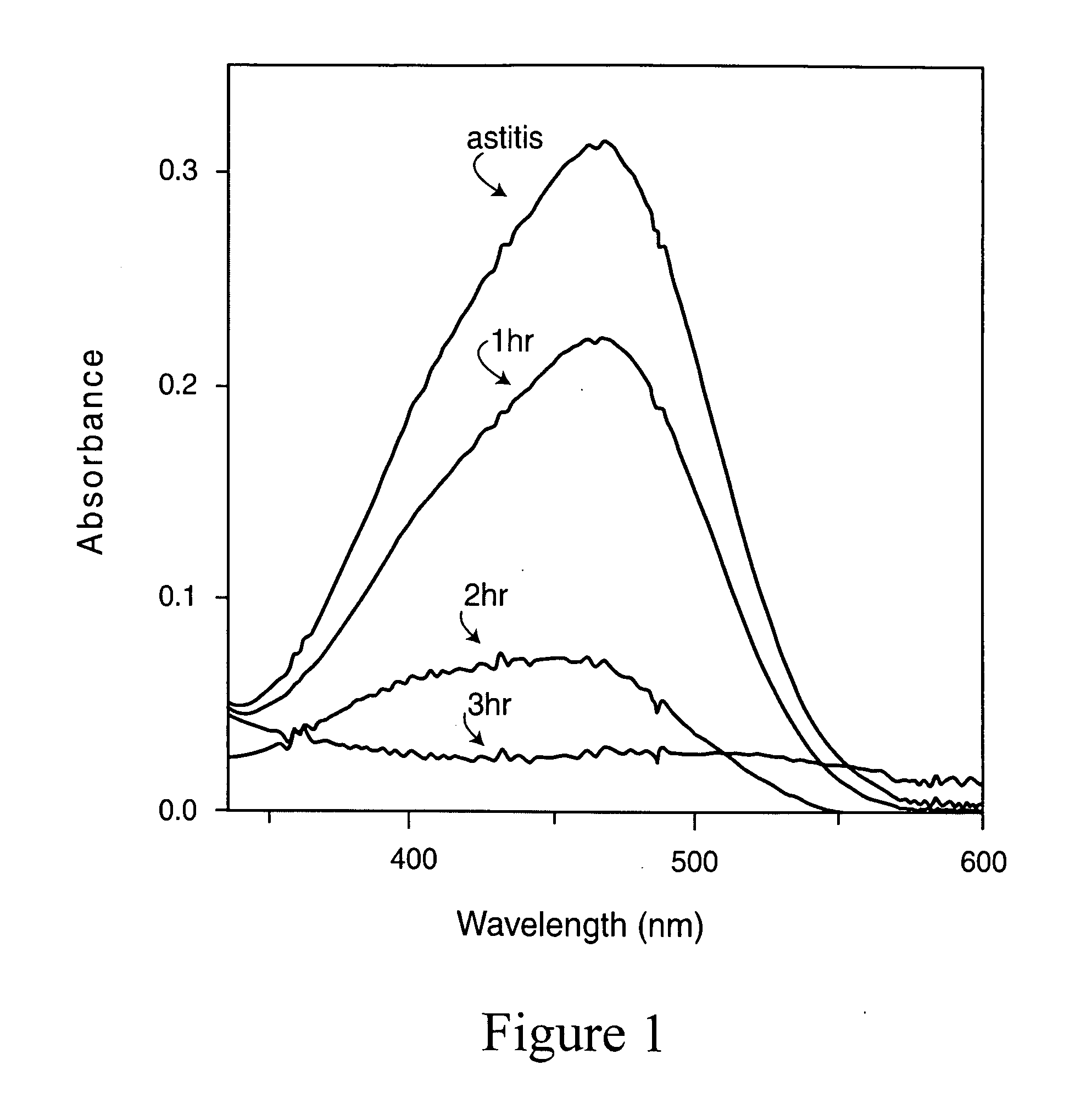
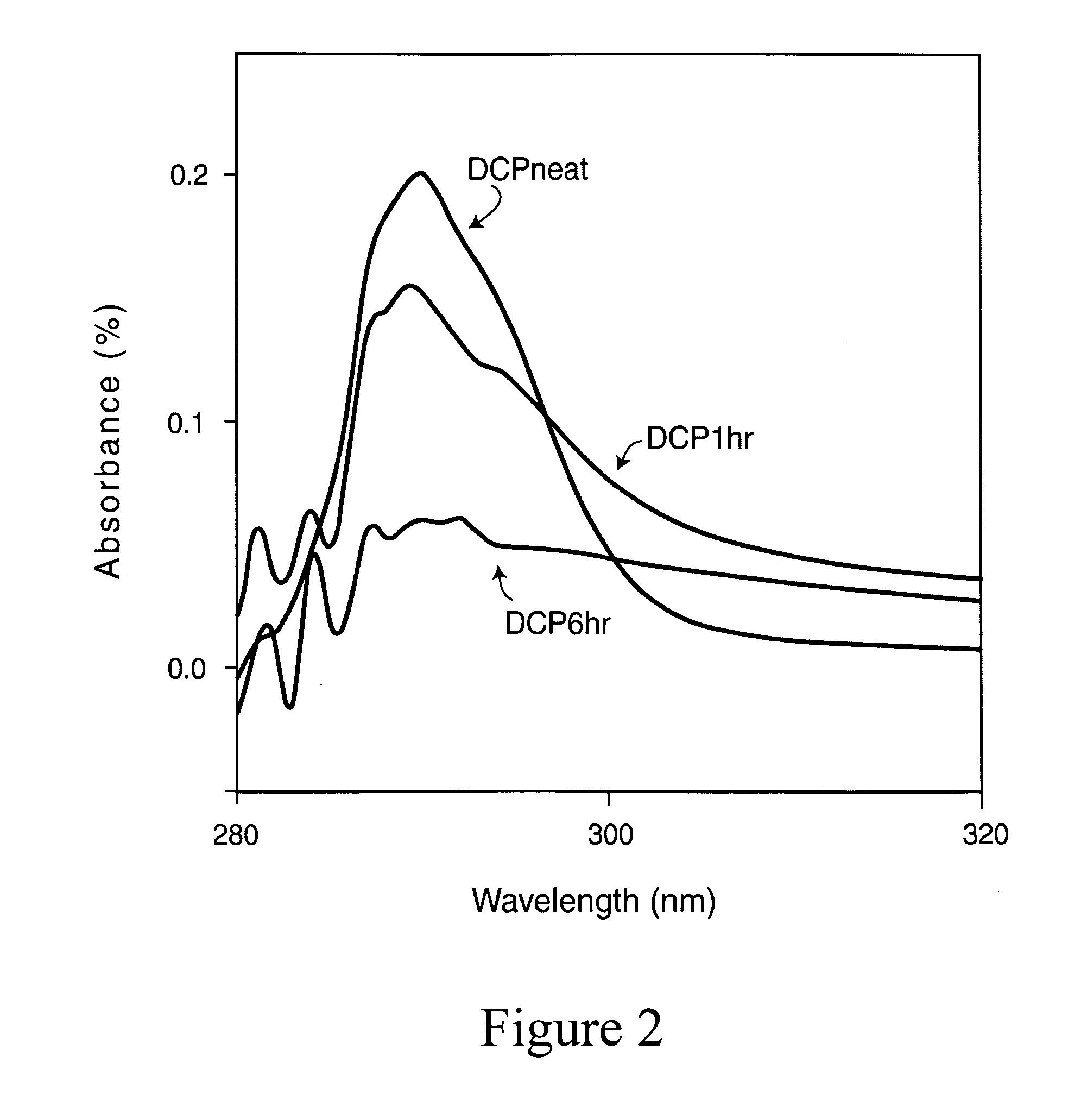
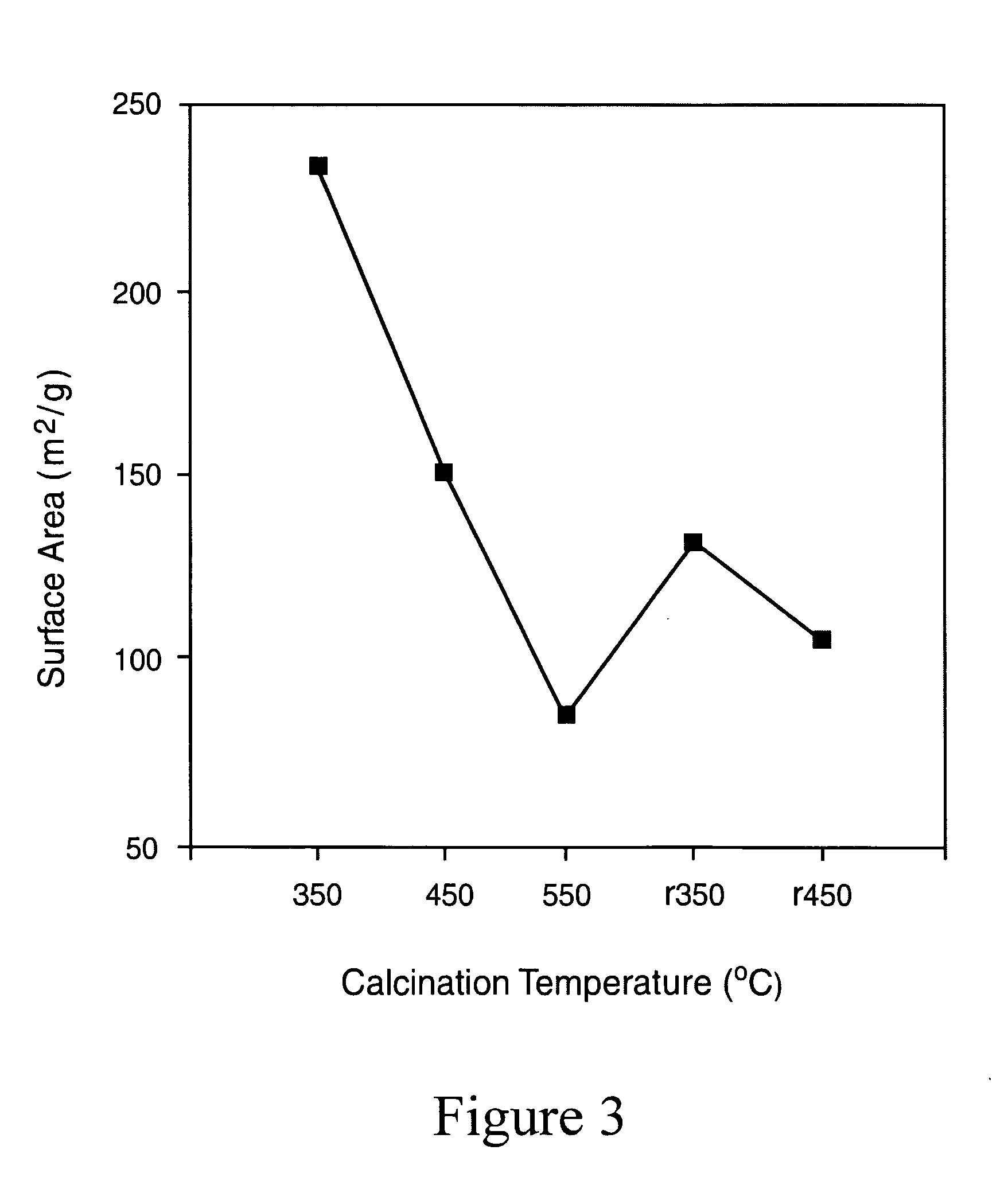
Magnetic nano-composite for contrast agent, intelligent contrast agent, drug delivery agent for simultaneous diagnosis and treatment, and separation agent for target substance
The present invention relates to water soluble magnetic nanocomposite using an amphiphilic compound. Specifically, the present invention relates to water soluble magnetic nanocomposite which may be not only used as a contrast agent for magnetic resonance imaging (MRI), an intelligent contrast agent for diagnosing cancer characterized by binding a tissue-specific binder ingredient, a drug delivery system for simultaneous diagnosis and treatment by polymerizing or enveloping drugs and binding a tissue-specific binder ingredient, but also used for separating a target substance using magnetism, and a process for preparing the same.
Owner:IND ACADEMIC CORP FOUND YONSEI UNIV
Doped titanium dioxide as a visible and sun light photo catalyst
ActiveUS20110266136A1High reactivityImprove stabilityGroup 1/11 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsPhotochemistryTitanium dioxide
Methods for preparing and using a photocatalyst are described. The catalyst is prepared by oxidation of a metal salt which has been doped in situ to form a photocatalyst active in visible light. The photocatalyst is used for degrading toxic and irritating compounds and infectious agents.
Owner:ENVIRONMENTAL PROTECTION AGENCY US
Ultrasonic and microwave methods for enhancing the rate of a chemical reaction and apparatus for such methods
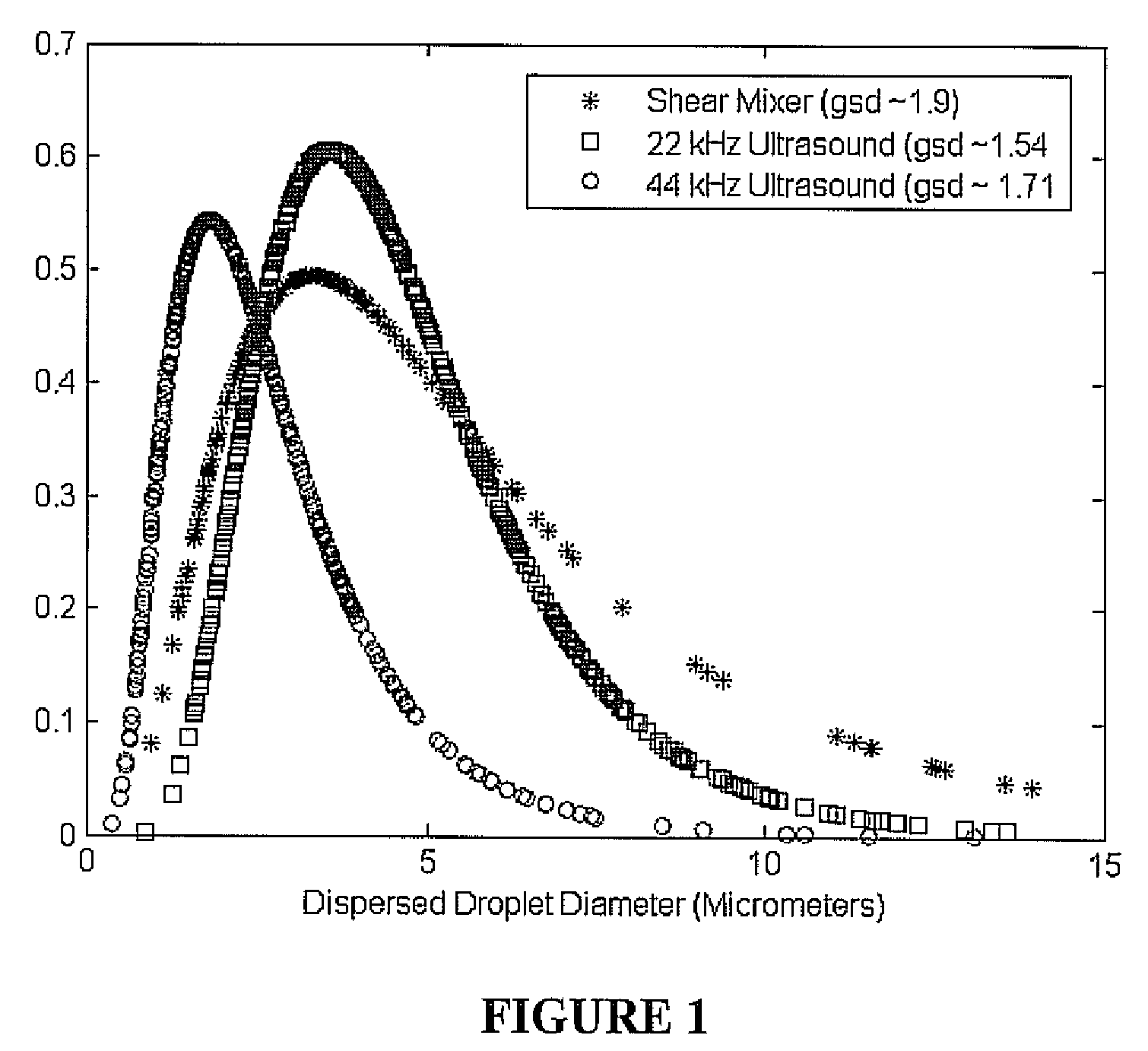
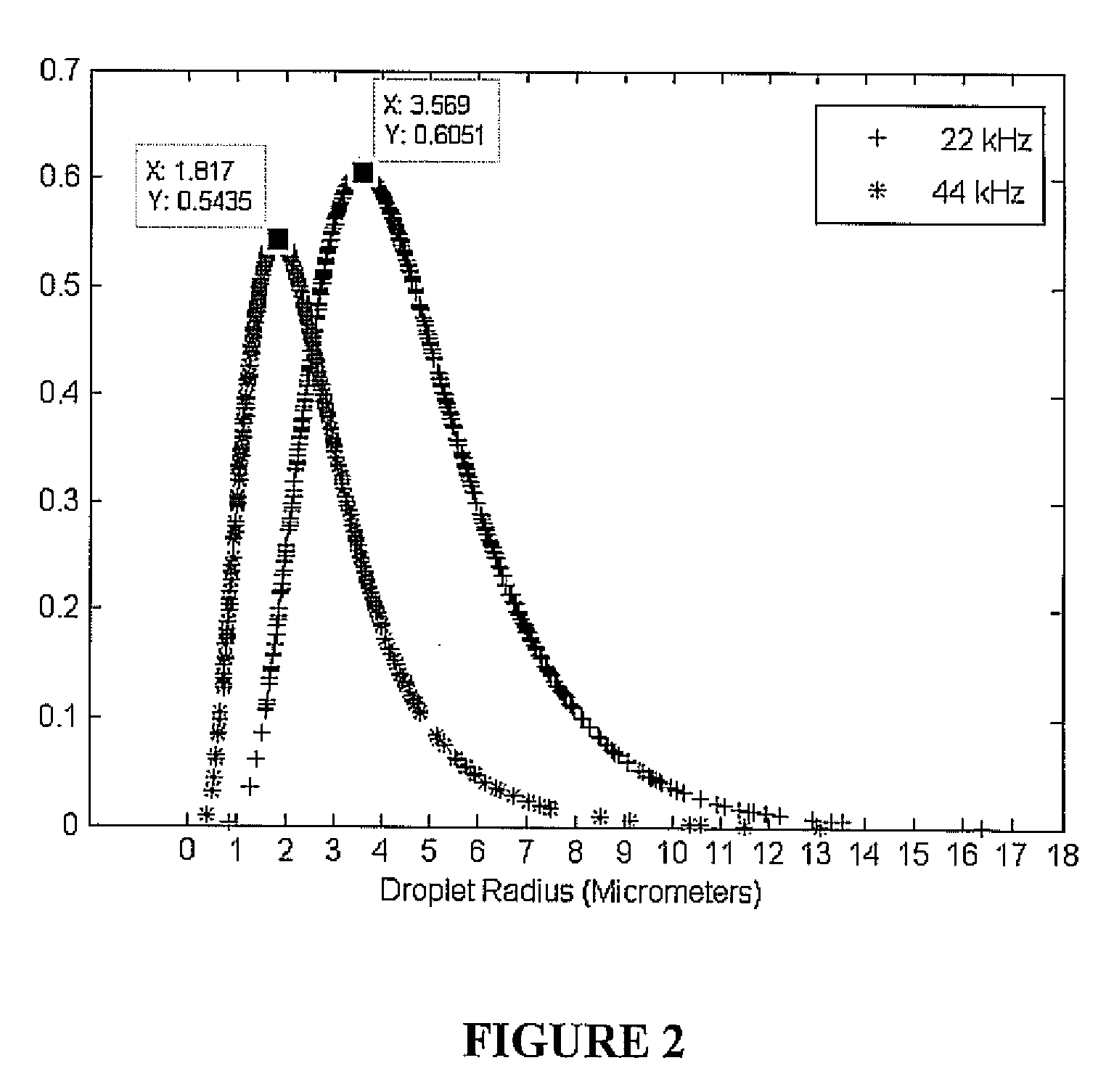
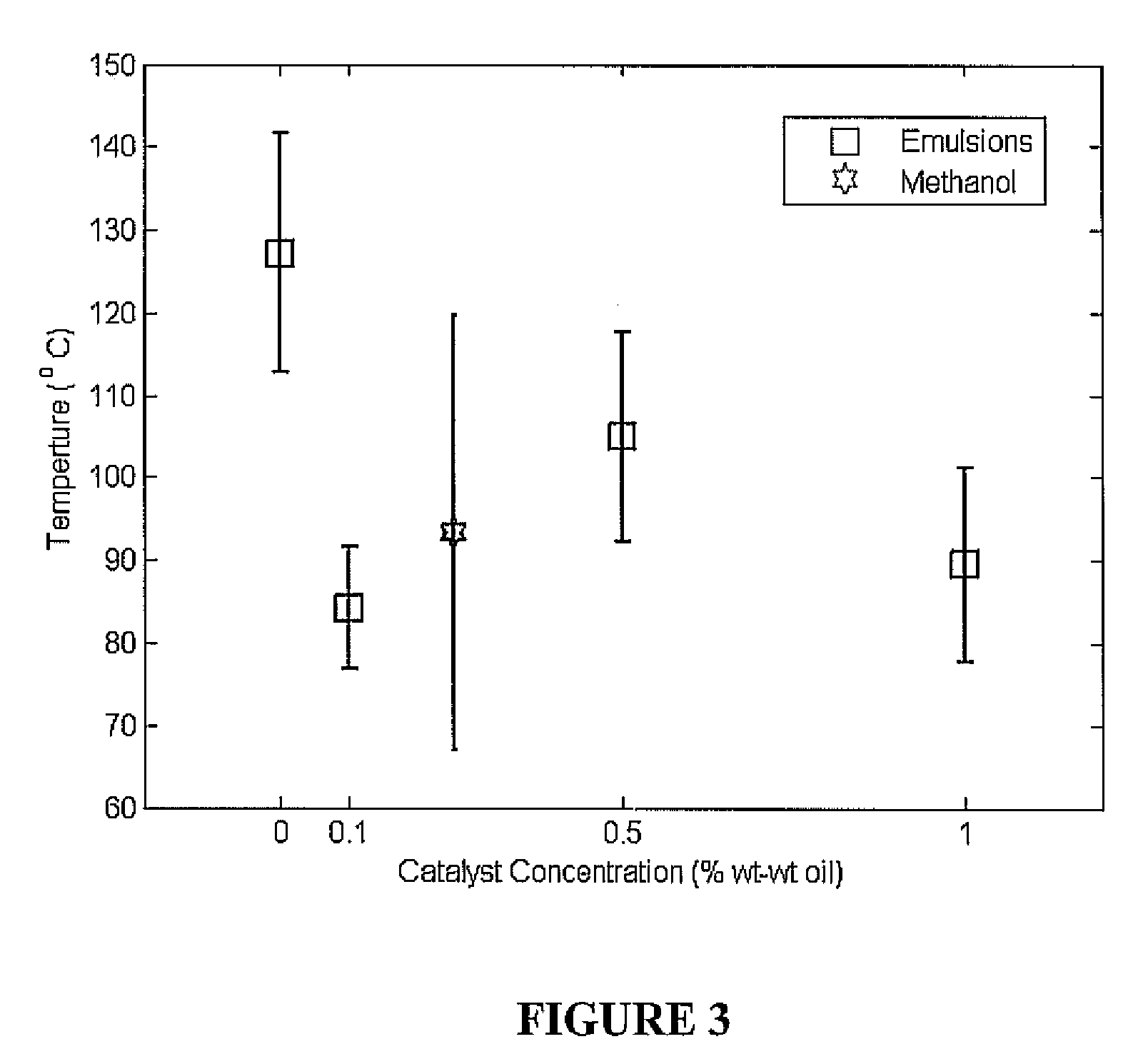
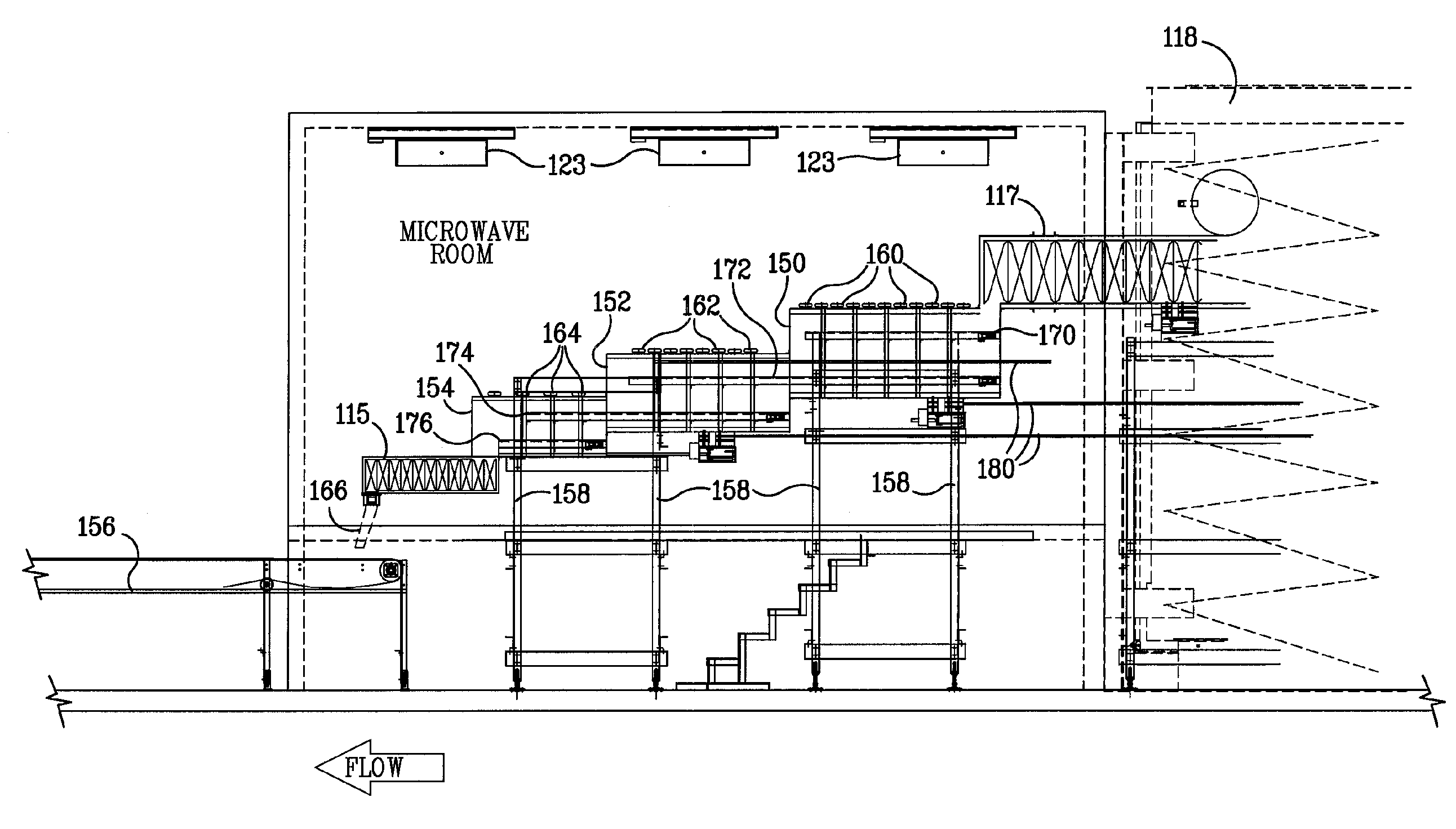
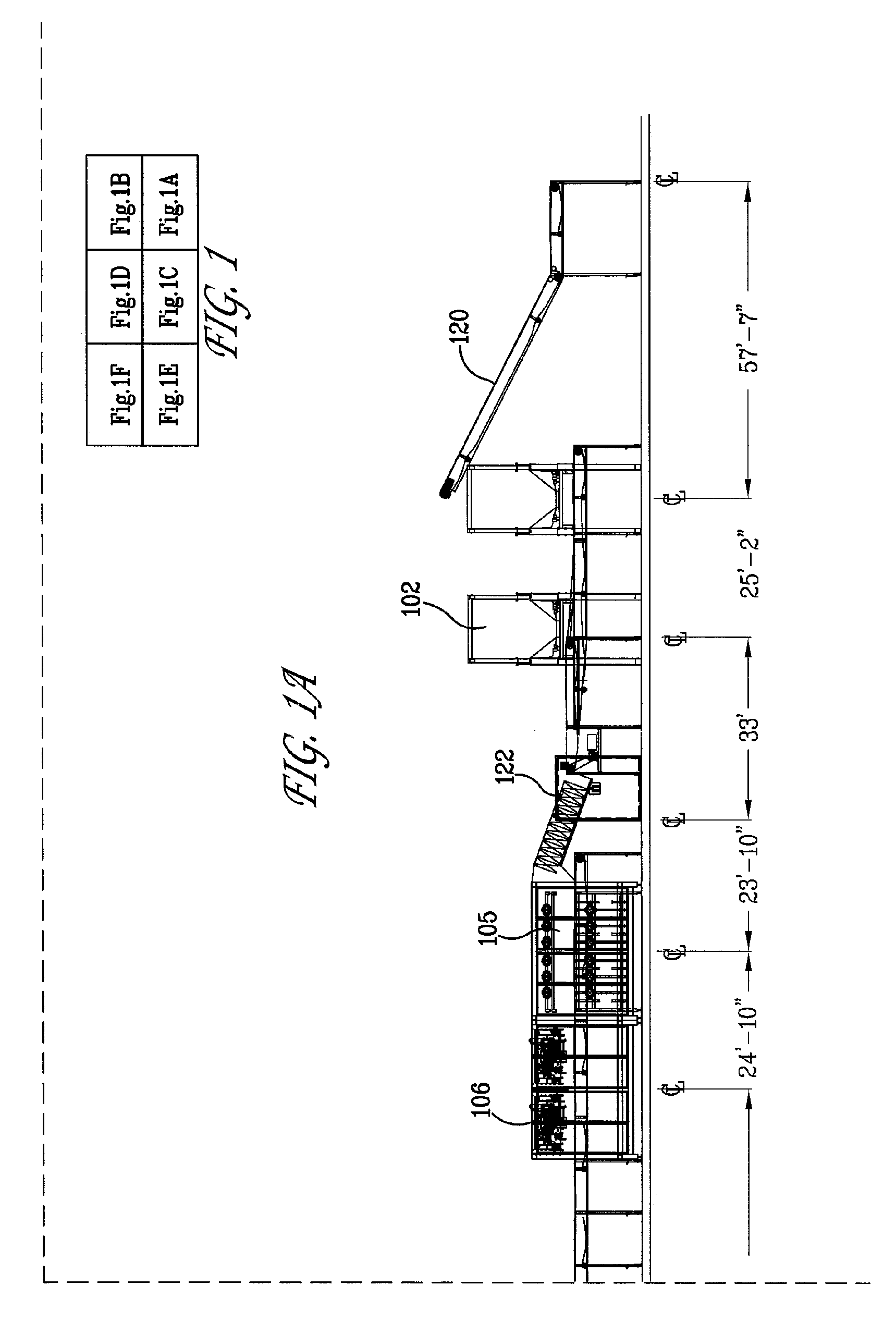
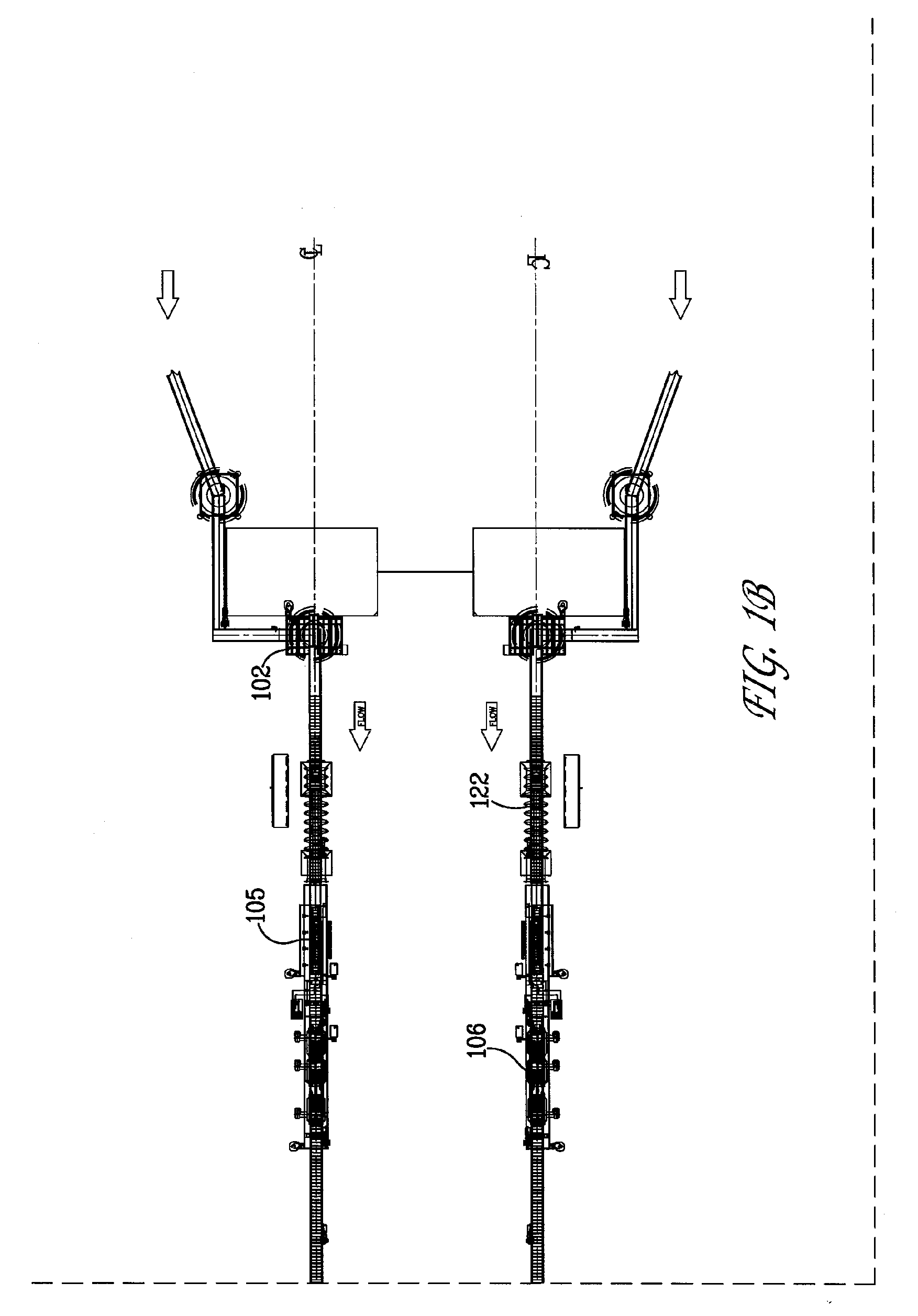
ActiveUS20090000941A1Increase the rate of chemical reactionsProduct can be usedFatty acid esterificationCarboxylic acid esters preparationChemical reactionMicrowave method
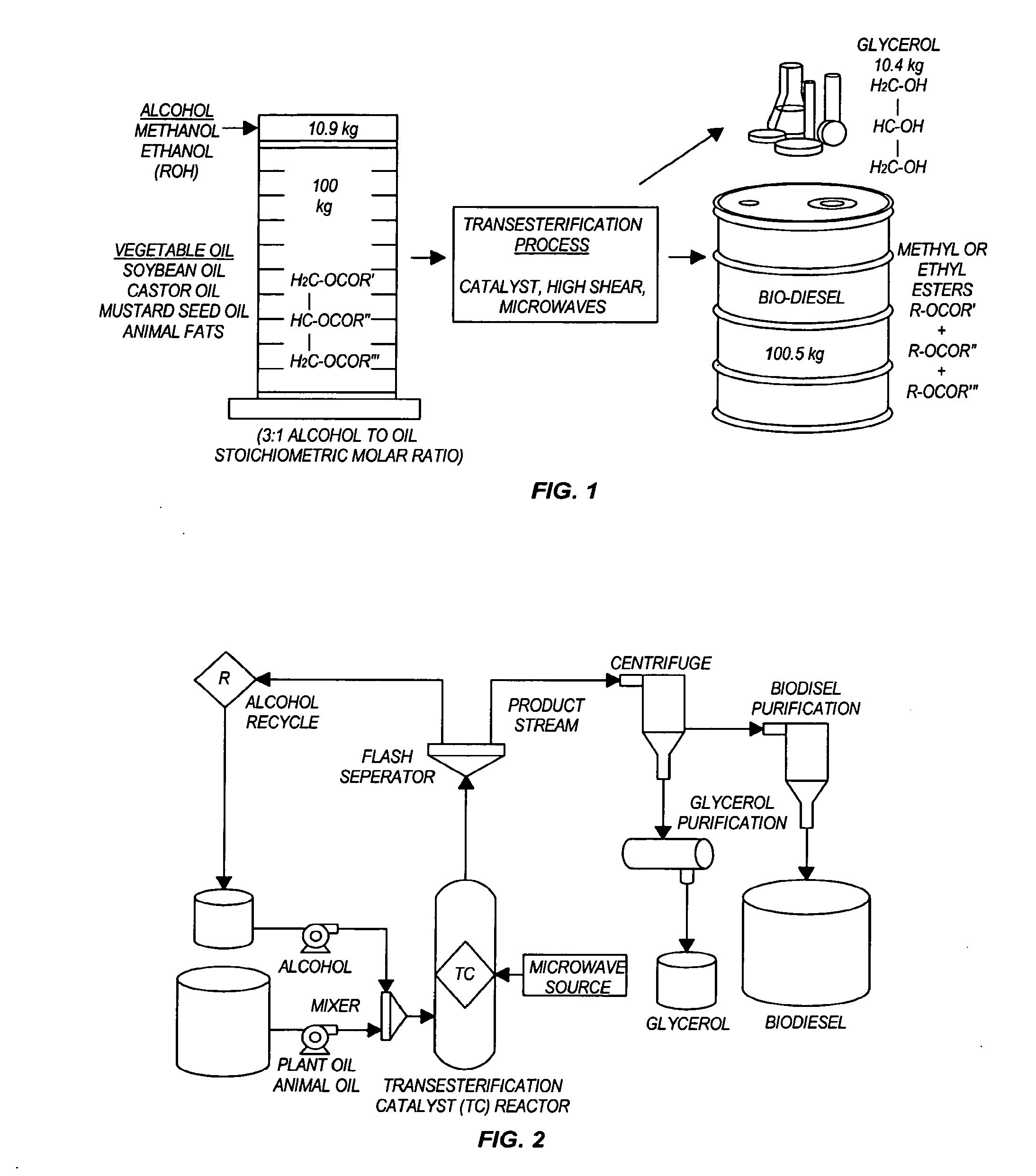
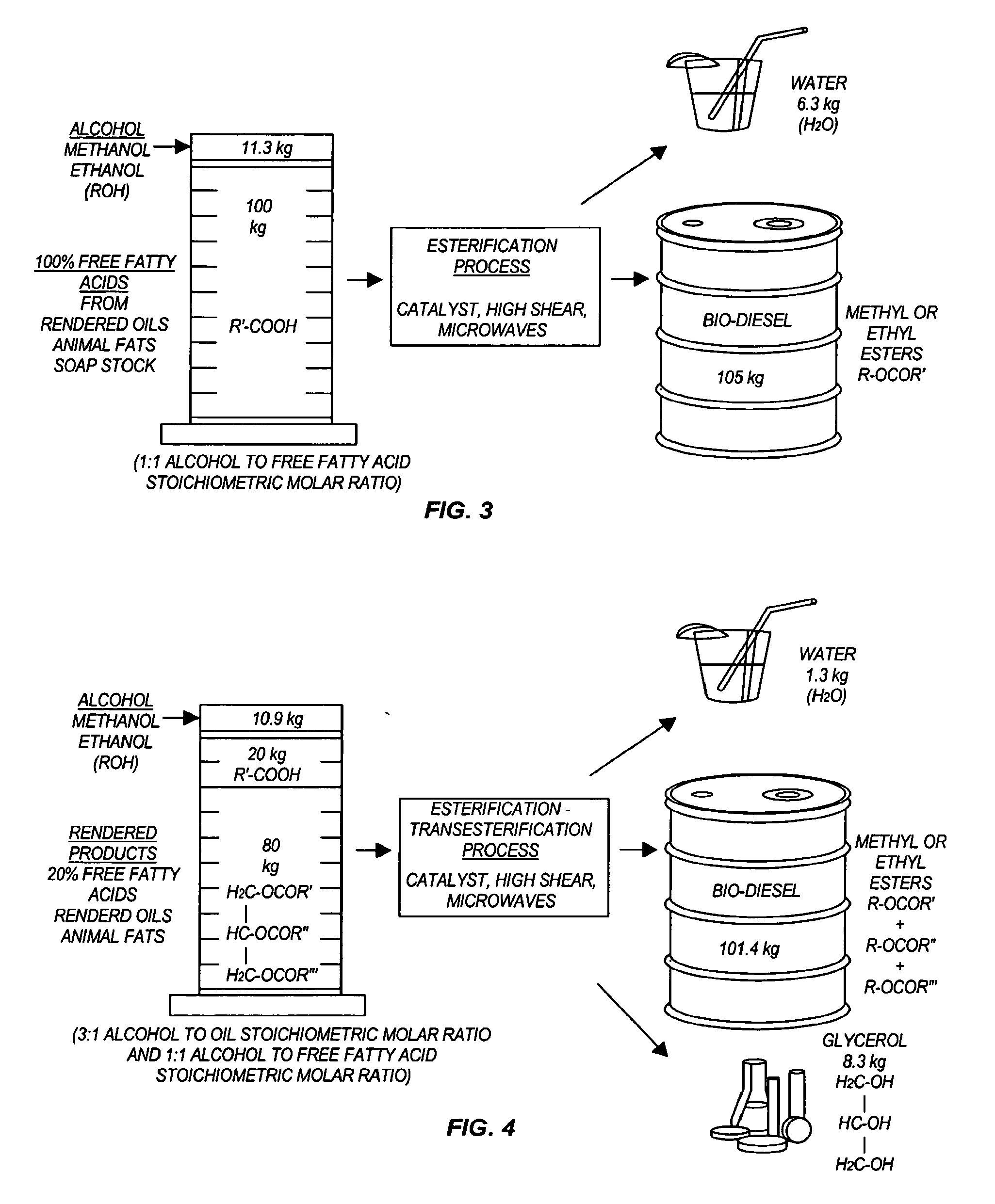
The invention relates generally to chemical reactions and processes, and in particular to a method for enhancing the rate of a chemical reaction and to apparatus for carrying out the method. The invention more particularly relates to methods and apparatus which utilize microwave and ultrasonic energy to enhance chemical reaction rates; and in specific instances, the invention relates to methods, processes and apparatus for the synthesis of biodiesel fuels. The methods, processes and apparatus of the invention are useful for the synthesis of biodiesel fuels; and also useful for production of reaction products of esterification and / or transesterification reactions including fatty acid alkyl esters.
Owner:PENN STATE RES FOUND
Precursors for cvd/ald of metal-containing films
InactiveUS20100018439A1Improve coordinationGroup 5/15 element organic compoundsGroup 8/9/10/18 element organic compoundsGas phaseElectron donor
Precursors useful for vapor phase deposition processes, e.g., CVD / ALD, to form metal-containing films on substrates. The precursors include, in one class, a central metal atom M to which is coordinated at least one ligand of formula (I):wherein:R1, R2 and R3 are each independently H or ogano moieties; andG1 is an electron donor arm substituent that increases the coordination of the ligand to the central metal atom M;wherein when Ga is aminoalkyl, the substituents on the amino nitrogen are not alkyl, fluoroalkyl, cycloaliphatic, or aryl, and are not connected to form a ring structure containing carbon, oxygen or nitrogen atoms. Also disclosed are ketoester, malonate and other precursors adapted for forming metal-containing films on substrates, suitable for use in the manufacture of microelectronic device products such as semiconductor devices and flat panel displays.
Owner:ENTEGRIS INC
Method for preparing conductive polymer dispersion, conductive polymer material made therefrom and solid electrolytic capacitor using the material
InactiveUS20130342967A1Short reaction timeReduce surface resistanceGroup 1/11 element organic compoundsSolid electrolytic capacitorsMicrowavePolymer science
The present invention provides a method for preparing a conductive polymer dispersion, including: adding a conductive compound, a polyanion, and an oxidant to a solvent; and polymerizing the conductive compound with microwaves. The present invention further provides a conductive polymer material made from the conductive polymer dispersion and a solid electrolyte capacitor using the conductive polymer material. Compared to a conventional method, the conductive polymer is prepared by the method of the present invention in a shorter time and environmental friendly. Moreover, the conductive polymer material made from the dispersion exhibits a high conductivity.
Owner:FAR EASTERN NEW CENTURY COPRRATION
Microwave-based recovery of hydrocarbons and fossil fuels
InactiveUS20080314730A1Group 1/11 element organic compoundsLiquid hydrocarbon mixture productionMicrowaveHydrocotyle bowlesioides
Owner:GORTRAGH DEV
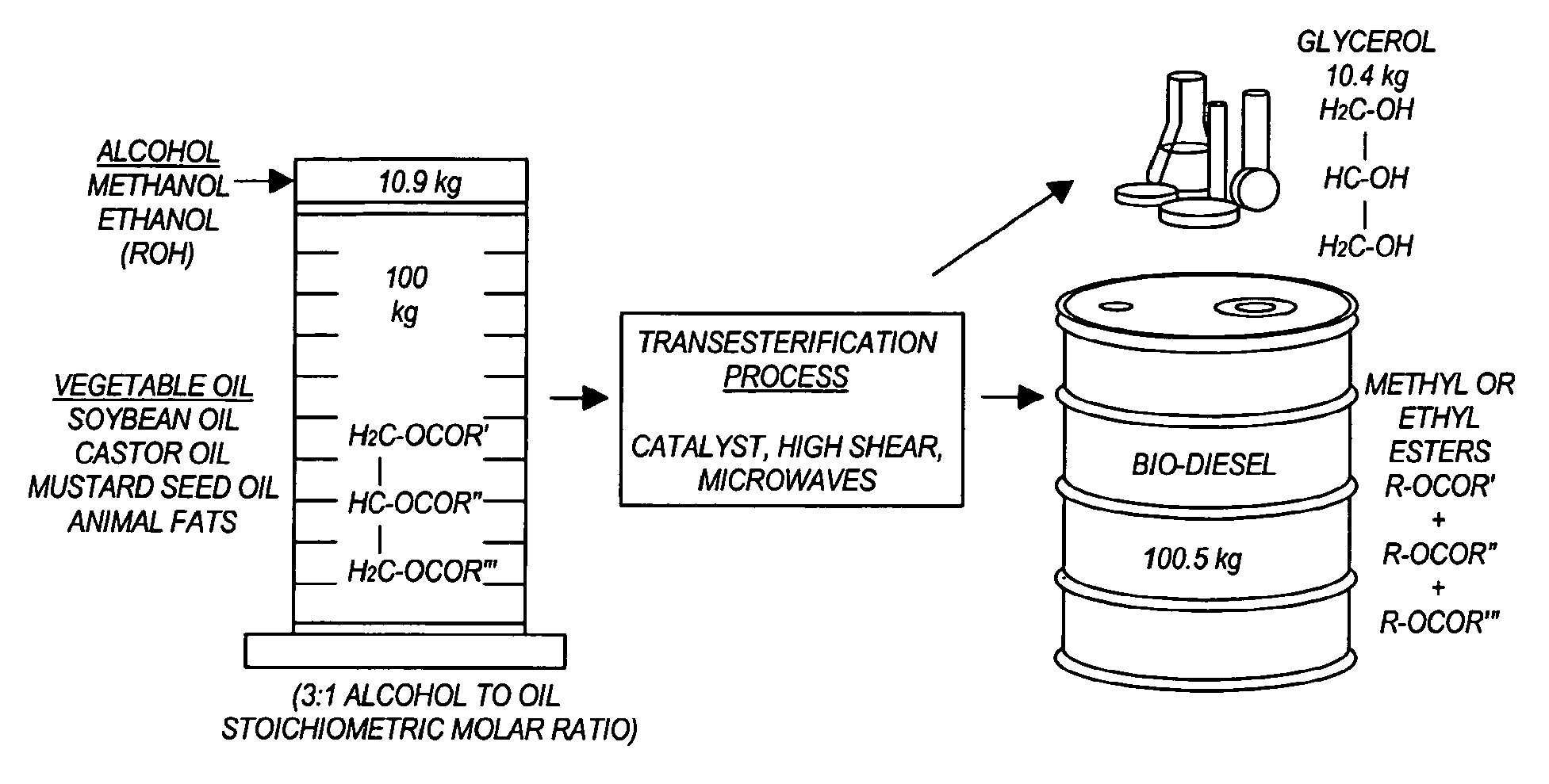
Methods for producing biodiesel
InactiveUS20100264015A1Fatty acid esterificationFatty acids production/refiningMicrowaveTransesterification
Transesterification, esterification, and esterification-transesterification (both one-step and two-step) for producing biofuels. The process may be enhanced by one or more of the following: 1) applying microwave or RF energy; 2) passing reactants over a heterogeneous catalyst at sufficiently high velocity to achieve high shear conditions; 3) emulsifying reactants with a homogeneous catalyst; or 4) maintaining the reaction at a pressure at or above autogeneous pressure. Enhanced processes using one or more of these steps can result in higher process rates, higher conversion levels, or both.
Owner:CARNEGIE MELLON UNIV
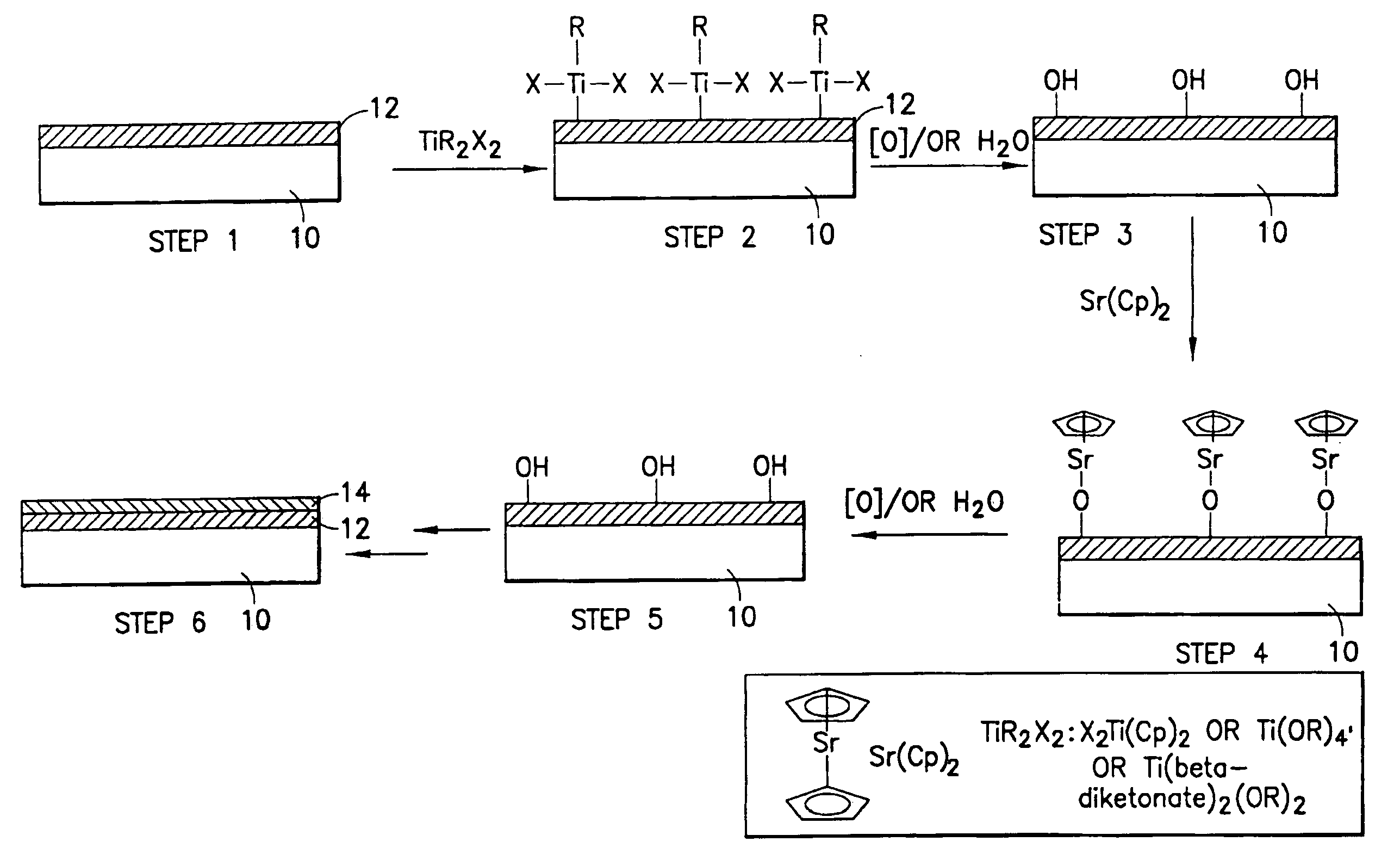
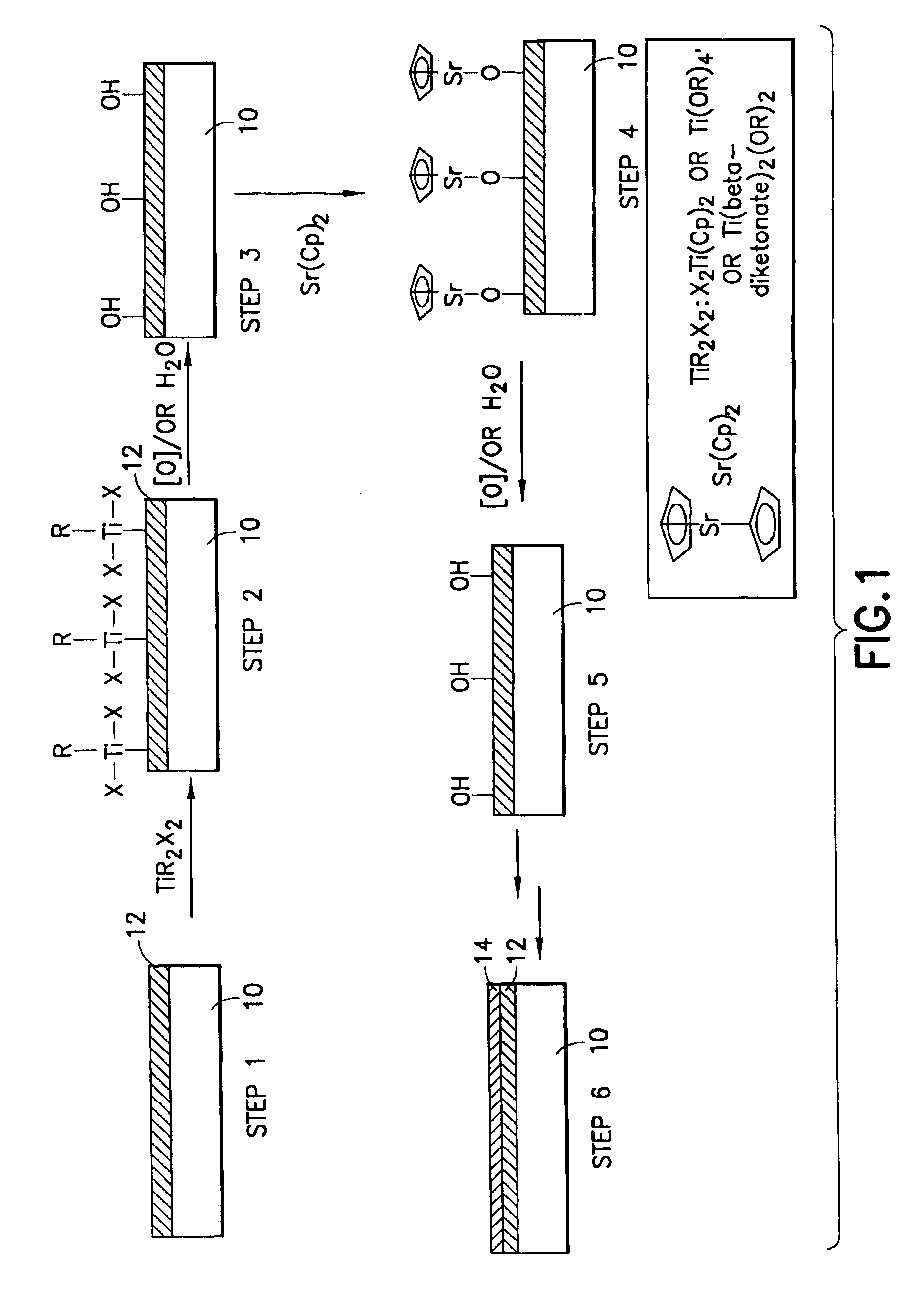
Precursor compositions for atomic layer deposition and chemical vapor deposition of titanate, lanthanate, and tantalate dielectric films
Barium, strontium, tantalum and lanthanum precursor compositions useful for atomic layer deposition (ALD) and chemical vapor deposition (CVD) of titanate thin films. The precursors have the formula M(Cp)2, wherein M is strontium, barium, tantalum or lanthanum, and Cp is cyclopentadienyl, of the formula (I), wherein each of R1-R5 is the same as or different from one another, with each being independently selected from among hydrogen, C1-C12 alkyl, C1-C12 amino, C6-C10 aryl, C1-C12 alkoxy, C3-C6 alkylsilyl, C2-C12 alkenyl, R1R2R3NNR3, wherein R1, R2 and R3 may be the same as or different from one another and each is independently selected from hydrogen and C1-C6 alkyl, and pendant ligands including functional group(s) providing further coordination to the metal center M. The precursors of the above formula are useful to achieve uniform coating of high dielectric constant materials in the manufacture of flash memory and other microelectronic devices.
Owner:ENTEGRIS INC
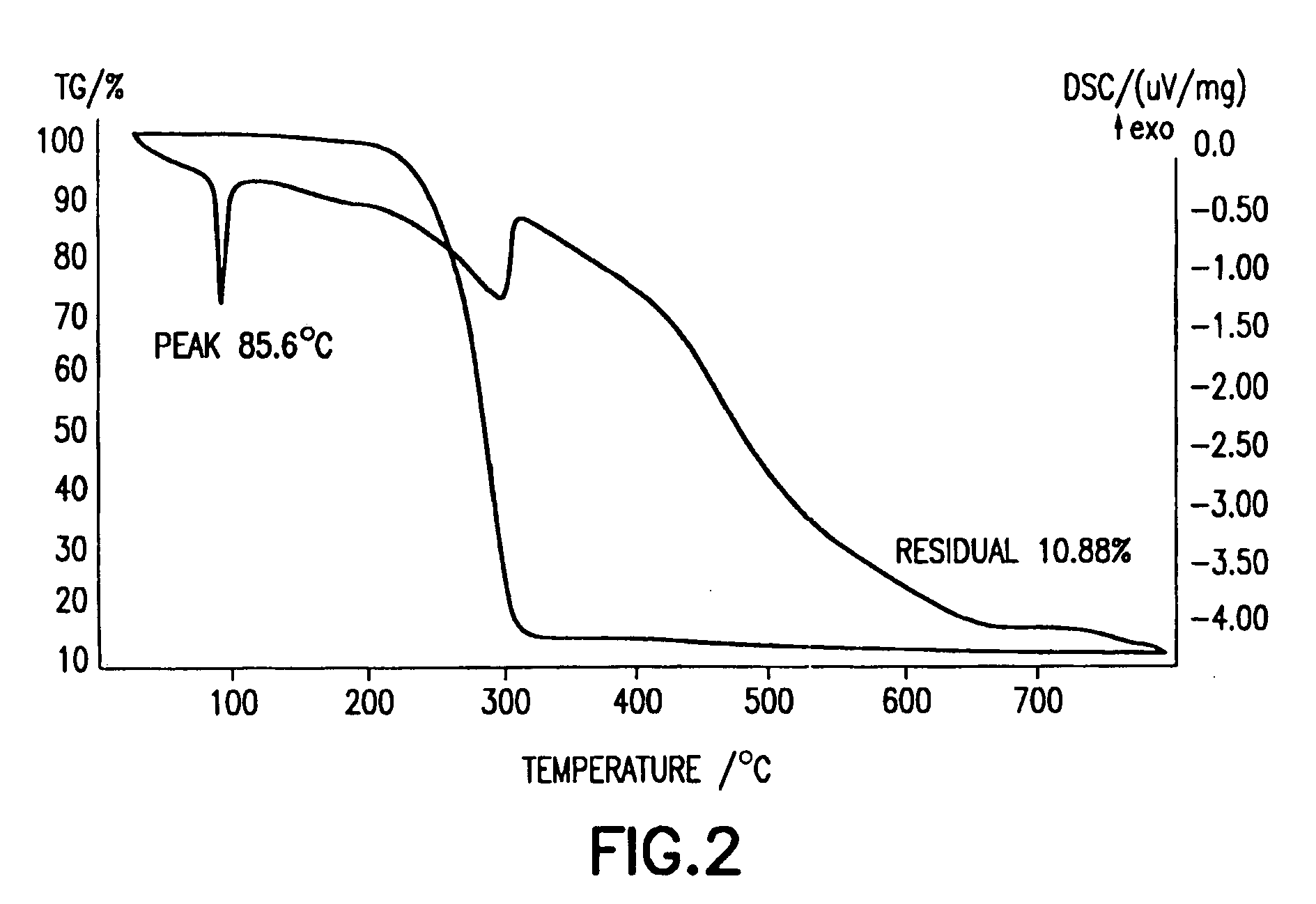
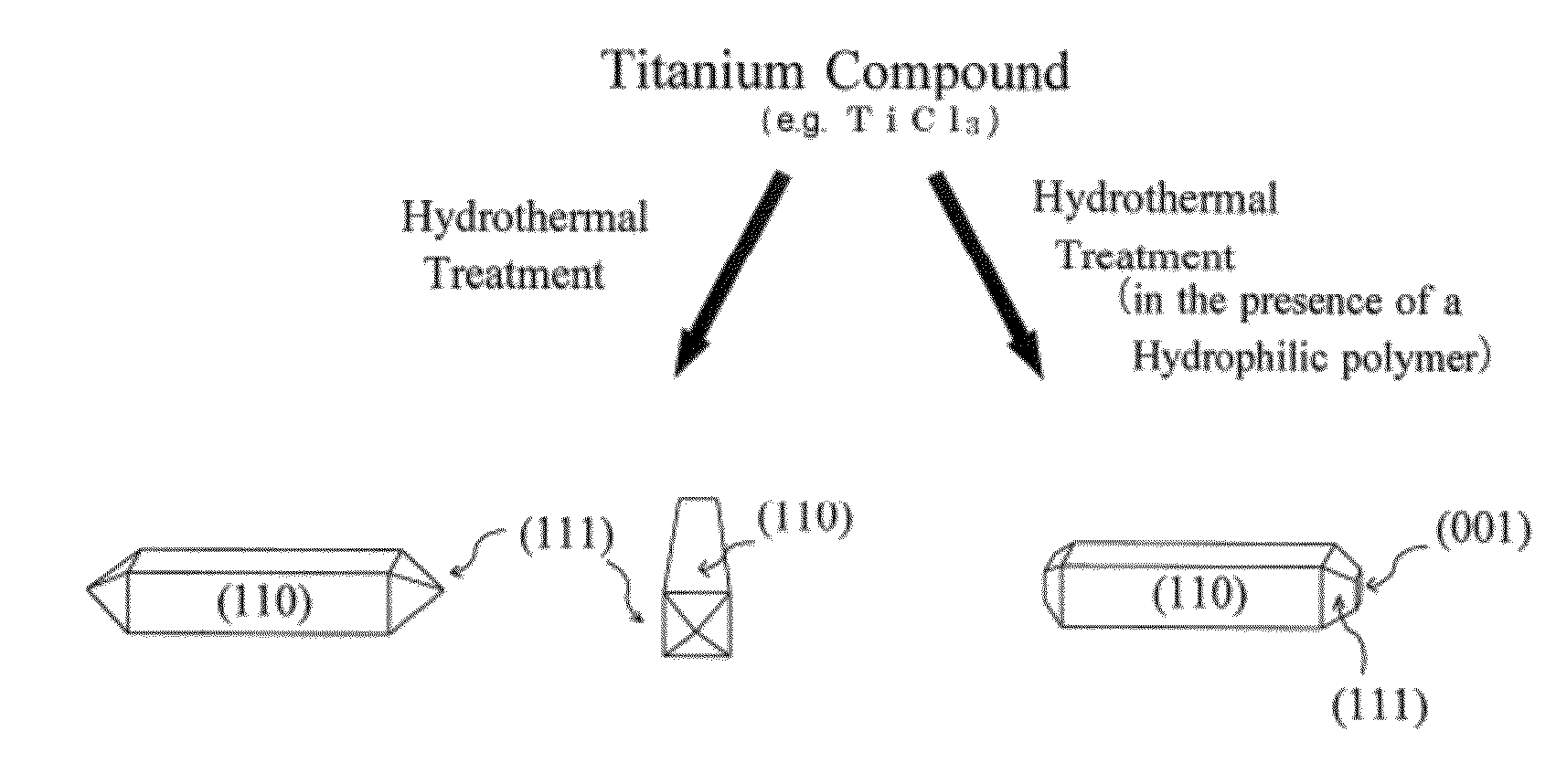
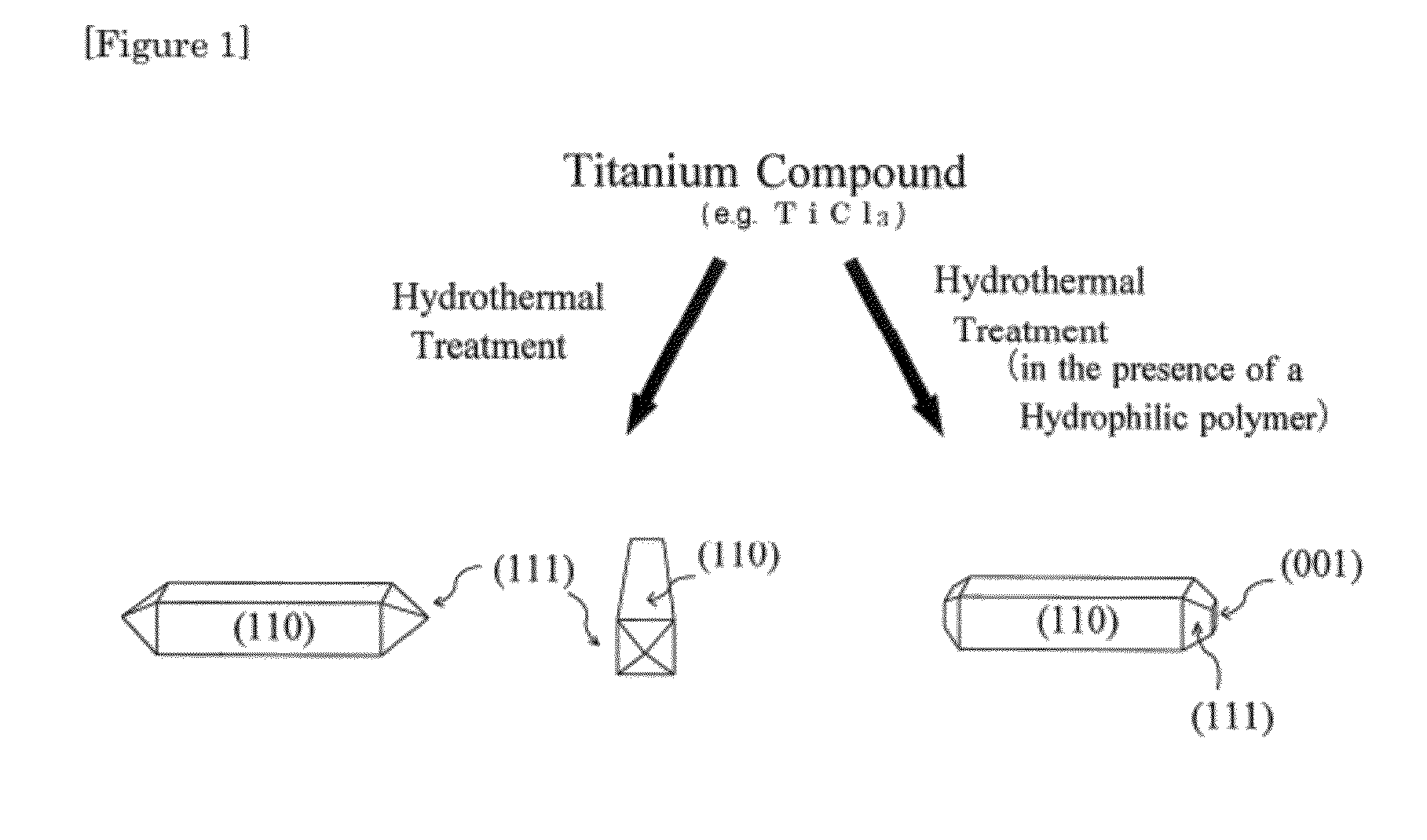
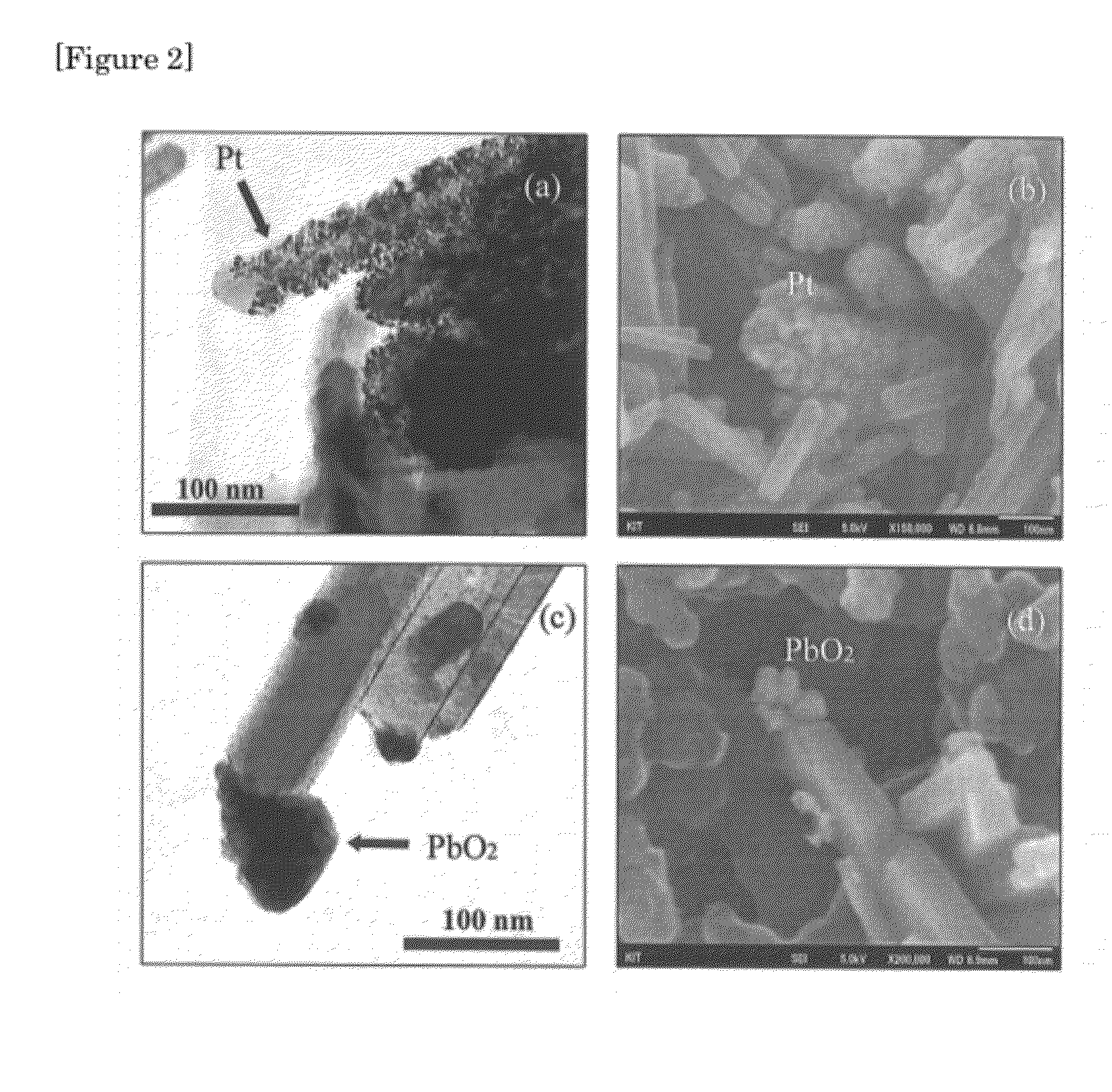
Rutile titanium dioxide nanoparticles each having novel exposed crystal face and method for producing same
InactiveUS20120132515A1Strong oxidation abilityEfficient of substanceMaterial nanotechnologyOrganic oxidationHydrophilic polymersOrganic compound
Provided are: novel rutile titanium dioxide nanoparticles each having a high photocatalytic activity; a photocatalyst including the rutile titanium dioxide nanoparticles; and a method for oxidizing an organic compound using the photocatalyst. The rutile titanium dioxide nanoparticles each have an exposed crystal face (001). The rutile titanium oxide nanoparticles may be produced by subjecting a titanium compound to a hydrothermal treatment in an aqueous medium in the presence of a hydrophilic polymer. A polyvinylpyrrolidone, for example, is used as the hydrophilic polymer. An organic compound having an oxidizable moiety can be oxidized with molecular oxygen or a peroxide under photoirradiation in the presence of the photocatalyst including the rutile titanium oxide nanoparticles.
Owner:DAICEL CHEM IND LTD
Polyphenylene host compounds
InactiveUS20110251401A1Solid-state devicesGroup 2/12 element organic compoundsOrganic light emitting deviceHost material
Owner:NITTO DENKO CORP
Deuterated Semiconducting Organic Compounds for Use in Light-Emitting Devices
InactiveUS20090295274A1Improve voltage stabilityLower turn-on voltageGroup 4/14 element organic compoundsGroup 1/11 element organic compoundsEnergy transferTransport layer
The present invention discloses deuterated semiconducting organic compounds. The deuterated semiconducting organic compounds comprise at least one partially or fully deuterated non-conjugated portion linked to the conjugated portion. The mentioned deuterated semiconducting organic compounds can be used in optoelectronic devices, such as light-emitting devices and photodiodes, with enhanced performance and lifetime. The deuterated semiconducting organic compounds of this application can be employed as emissive layer, charge-transporting layer, or energy transfer material in organic light-emitting devices.
Owner:HWANG KUO CHU
Plasma reactor for carrying out gas reactions and method for the plasma-supported reaction of gases
ActiveUS8636960B2Promote activationImprove throughputGroup 3/13 element organic compoundsMicrowave heatingPlasma reactorPlasma chamber
A device for carrying out gas reactions, comprising a plasma reactor with a through-flow of gases which has a, particularly cylindrical, plasma chamber, wherein flow-forming elements for forming a flow of gases are arranged before and / or in and / or after the plasma reactor in order to form a gas stream within the plasma chamber such that at least one, particularly central, zone in the gas flow is formed which is flow-reduced. A method for carrying out gas reactions is also provided.
Owner:IPLAS
Organic electrolysis reactor for performing an electrolytic oxidation reaction and method for producing a chemical compound by using the same
Disclosed is an organic electrolysis reactor for performing an electrolytic oxidation reaction of a system comprising a substrate and a reductant, comprising: a casing; an anode which comprises an anode active material and which is ion-conductive or active species-conductive; a cathode which comprises a cathode active material and which is ion-conductive or active species-conductive; and means for applying a voltage between the anode and the cathode, wherein the means for applying a voltage is disposed in the outside of the casing and connected to the anode and the cathode, wherein the anode and the cathode are disposed in spaced relationship in the casing to partition the inside of the casing into an intermediate compartment between the anode and the cathode, and an anode compartment on the outside of the anode. Also disclosed is a method for producing a chemical compound by performing an electrolytic oxidation reaction of a system comprising a substrate and a reductant, using the organic electrolysis reactor mentioned above.
Owner:ASAHI KASEI KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com