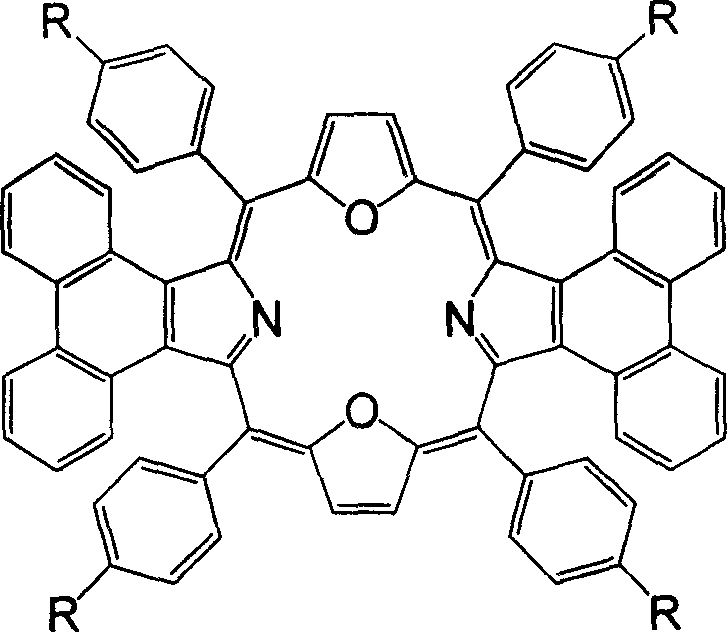
Synthesis of middle position-tetraaryldiphenanthrene dioxyporphyrin derivative and application thereof
A technology of tetraaryl diphenanthrene and dioxoporphyrin, which is applied in the fields of luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of many by-products, low yield, difficult purification, etc., and improve the absorption efficiency , Molar absorptivity increases, and the effect of improving conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
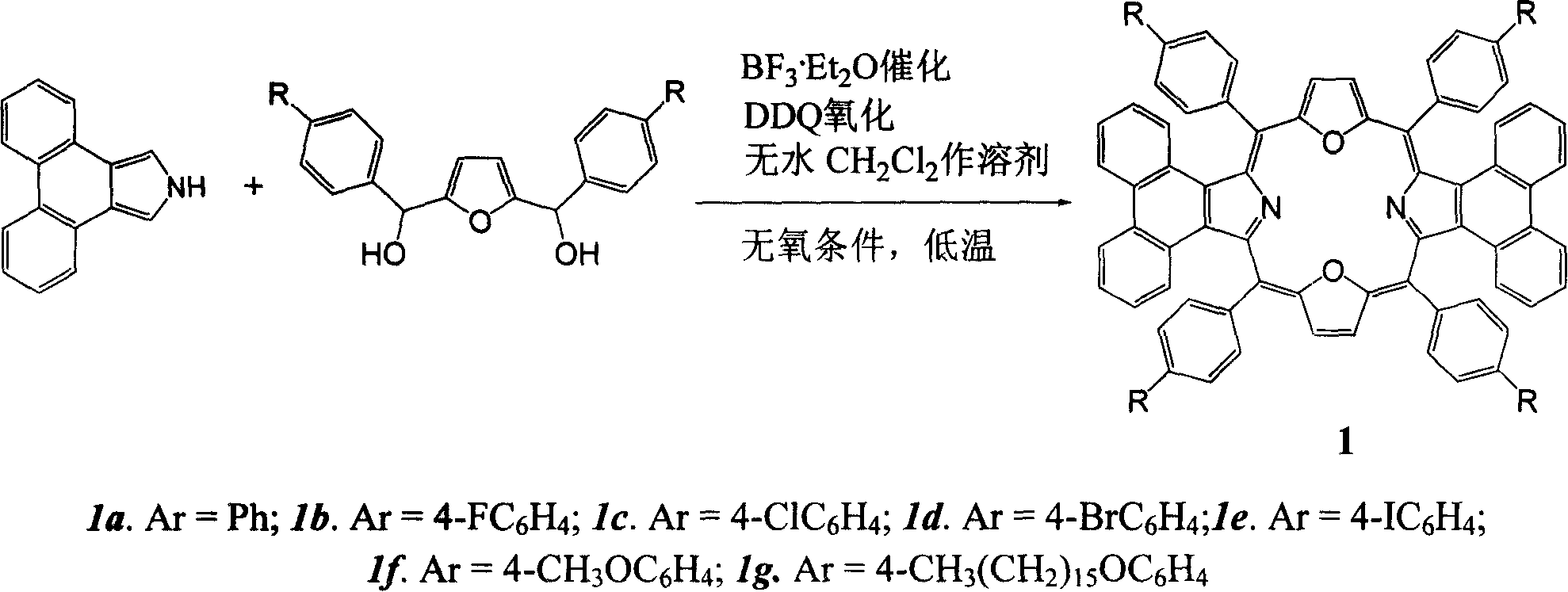
[0031] Example 1: 5,10,15,20-tetraphenyl-diphenanthrene[9,10-b:9,10-l]-22,24-dioxoporphyrin (1a) was synthesized in a 250ml circle Add 1mmol (278mg) 2,5-bis(phenylhydroxymethyl)furan, 1mmol (217mg) phenanthropyrrole and 60ml anhydrous dichloromethane into the bottom flask, put a magnet and start stirring, and react under the protection of argon. Put the bottle in a cryogenic device and keep it away from light, control the reaction temperature at -40-±5°C, add a total of 80 μl of BF 3 ·Et 2 O, after making it react at low temperature for 2 hours, allow it to naturally warm up to room temperature and continue the reaction for 48 hours. 1.0 mmol DDQ (227 mg) was added to the reaction solution, and reacted for 1 hour. The solvent was distilled off under reduced pressure for chromatographic separation, and dark green crystals were obtained after recrystallization from methanol and chloroform. Yield: 31.9%; melting point: >250°C; MALDI-TOF MASS cacd for C 68 h 40 N 2 o 2 917....
Embodiment 2
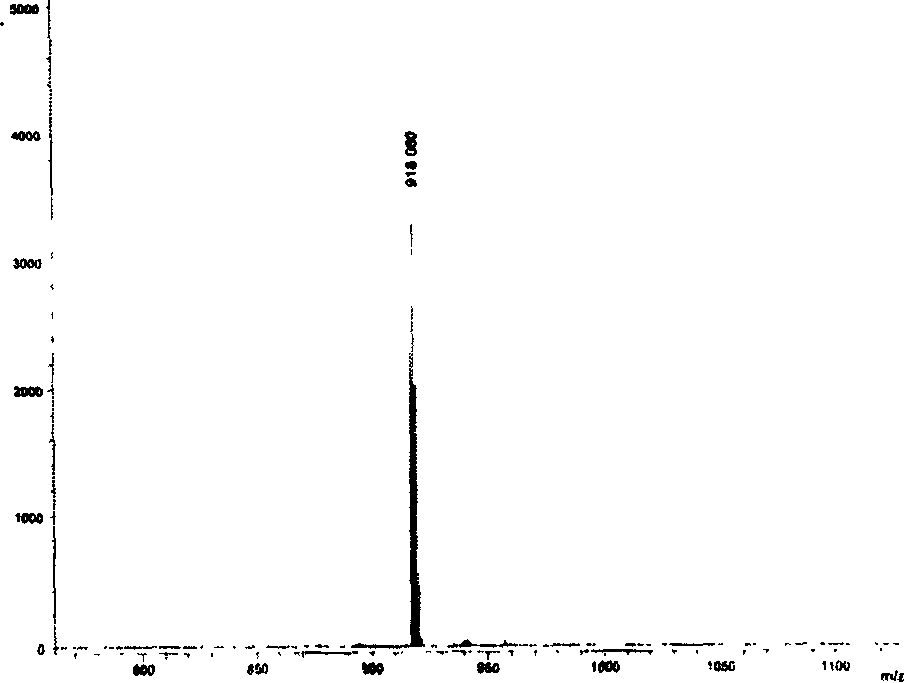
[0032] Example 2: 5,10,15,20-tetrakis(4-fluorophenyl)-diphenanthrene[9,10-b:9,10-l]-22,24-dioxoporphyrin (1b) The synthetic preparation method is the same as in Example 1, and the yield: 15.9%. Melting point: >250℃; MALDI-TOF MASS cacd for C 68 h 36 f 4 N 2 o 2 989.02, found: (M+H + )(Figure 5); 1 H-NMR (d 6 -DMSO) δ7.10-7.13 (4H, m), 7.43 (4H, s), 7.65 (8H, m), 7.83-7.85 (4H, m), 8.27 (4H, s); 8.63-8.64 (8H, m), 8.75 (4H, s); UV-vis (CHCl 3 )λmax,nm(ε×10 -5 )577 (0.715), 732 (0.0442), 808 (0.151) (Figure 6).
Embodiment 3
[0033] Example 3: 5,10,15,20-tetrakis(4-chlorophenyl)-diphenanthrene[9,10-b:9,10-l]-22,24-dioxoporphyrin (1c) The synthetic preparation method is the same as in Example 1, and the yield: 10.5%. Melting point: >250℃; MALDI-TOF MASS cacd for C 68 h 36 Cl 4 N 2 o 2 1054.84, found: (M+H + ) (Figure 7); 1 H-NMR (d 6 -DMSO) δ7.15-7.18 (8H, m), 7.48-7.51 (4H, m), 7.87-7.89 (8H, m), 8.32 (4H, s), 8.70-8.72 (8H, m), 8.82 ( 4H, s); UV-vis (CHCl 3 )λmax,nm(ε×10 -5 )577 (0.715), 732 (0.0442), 808 (0.151) (Figure 8).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com