Preparation method and application of 7-position vinyl substituted osmapentalyne
A technology of osmopentyne and vinyl, which is applied in the field of preparation of osmopentyne substituted by vinyl at the 7th position, and can solve problems such as limited synthesis methods and limited structures of final products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
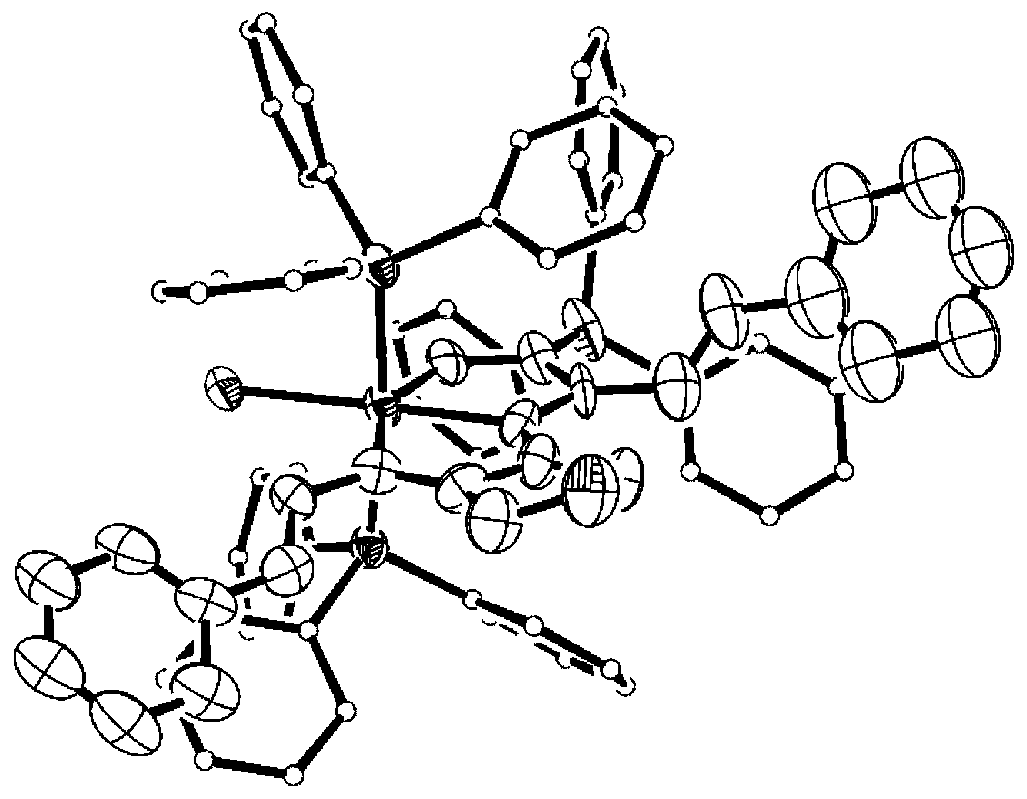
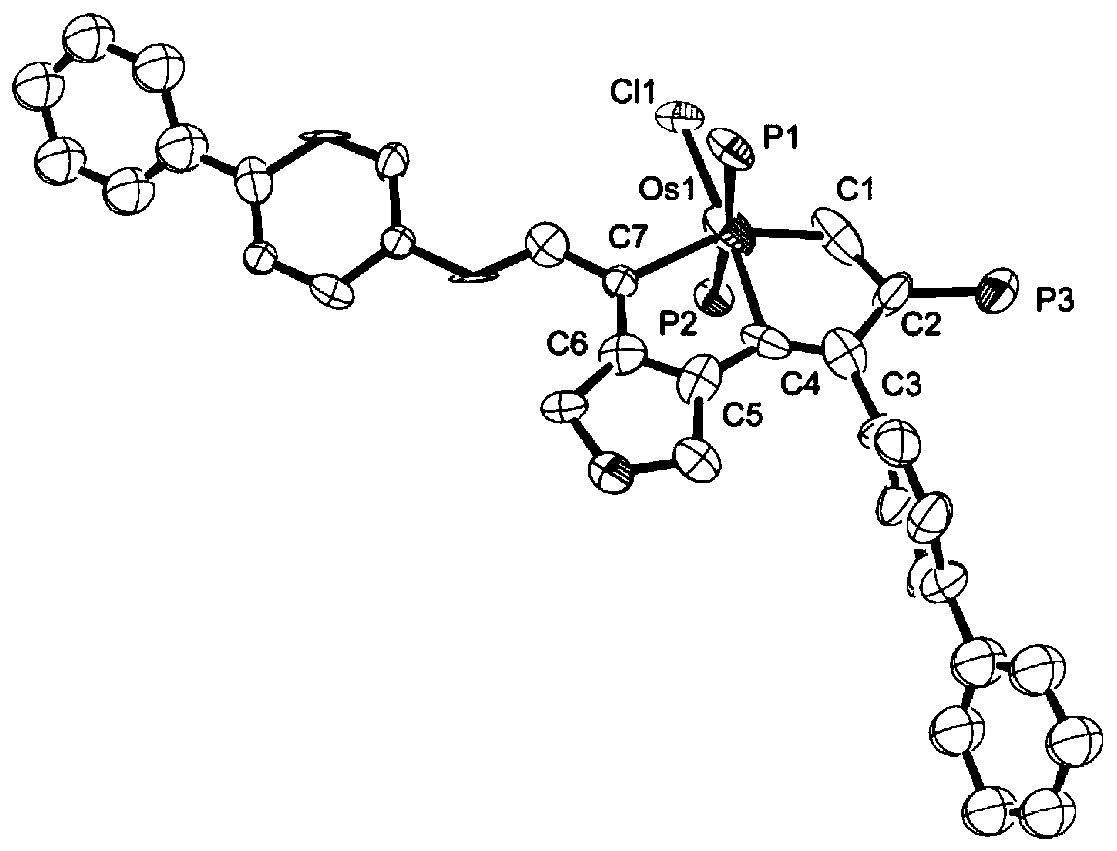
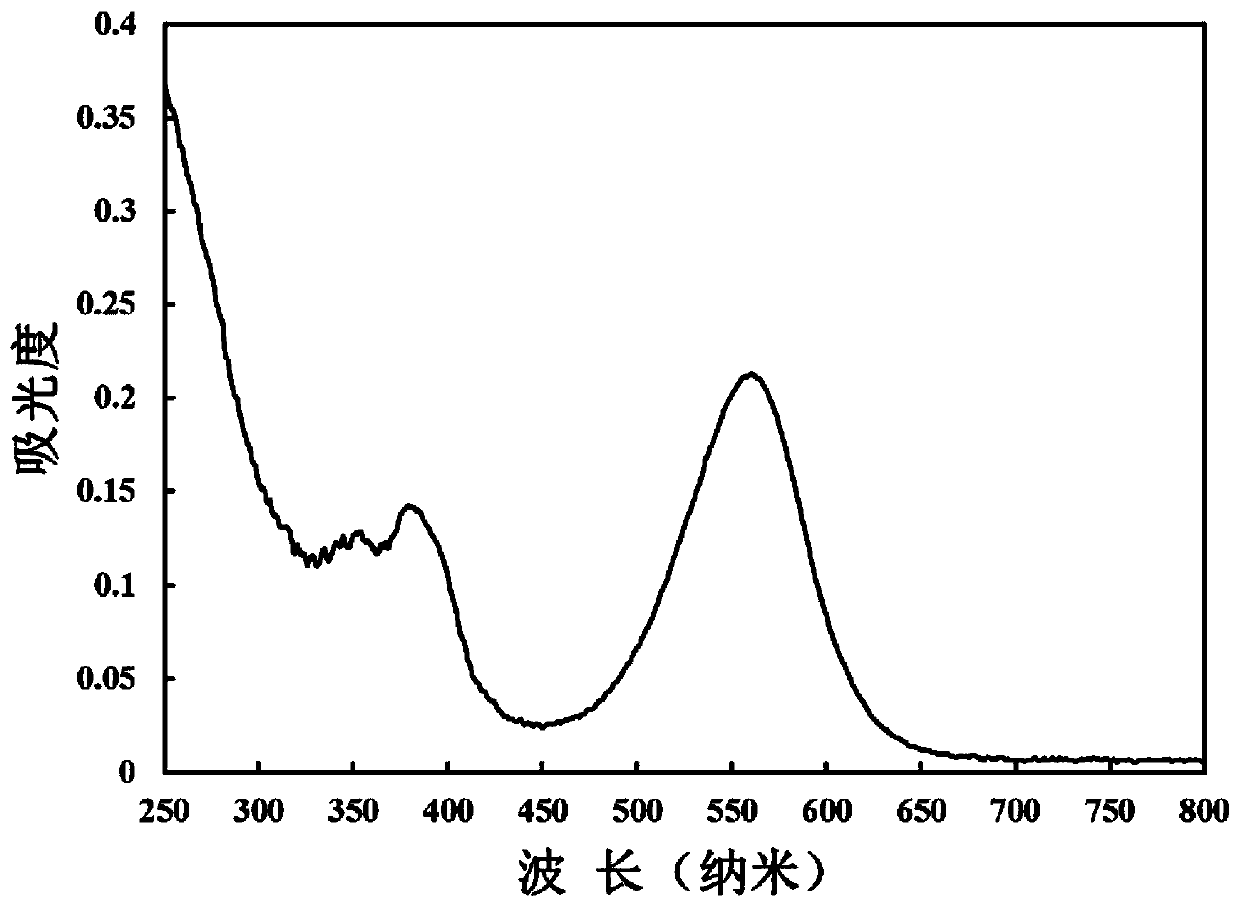
Image
Examples
Embodiment 1
[0121]
[0122] [Os] in the above formula is OsCl(PPh 3 ) 2 . Among them, HCl·Et 2 O is diethyl ether hydrochloride (purchased from Sarn Chemical Technology (Shanghai) Co., Ltd., the brand name is A01W8101821000), (Phenylacetylene, purchased from Bailingwei Technology Co., Ltd., the trade name is 445058), and the DCM used is dichloromethane redistilled solvent.
[0123] Preparation of compound III-1
[0124] Under a nitrogen atmosphere, under magnetic stirring, dissolve osmalpentyne compound I-4 (0.5mmol) and phenylacetylene II-1 (0.5mmol) in 50mL of dichloromethane, and quickly add 2mol / L hydrochloric acid Diethyl ether solvent 3mL, reacted at room temperature for 2h, concentrated, neutral alumina column chromatography (eluent: dichloromethane / methanol = 20 / 1 volume ratio), obtained 0.48mmol purple solid compound III-1, yield: 96%. (The yield of compound III-1 is the molar amount of compound III-1 / molar amount of ospentyne compound I-4×100%).
[0125] The NMR and h...
Embodiment 2
[0128] Method A.
[0129]
[0130] [Os] in the above formula is OsCl(PPh 3 ) 2 . Among them, HCl·Et 2 O is diethyl ether hydrochloride (purchased from Sarn Chemical Technology (Shanghai) Co., Ltd., the brand name is A01W8101821000), (Phenylacetylene, purchased from Bailingwei Technology Co., Ltd., the trade name is 445058), and the DCM used is dichloromethane redistilled solvent.
[0131] Preparation of Compound III-2
[0132] Under nitrogen atmosphere, under magnetic stirring, dissolve osmalpentyne compound IV-5 (0.5mmol) and phenylacetylene II-1 (0.5mmol) in 50mL of dichloromethane, and quickly add 2mol / L hydrochloric acid Diethyl ether solvent 3mL, reacted at room temperature for 2h, concentrated, neutral alumina column chromatography (eluent: dichloromethane / methanol = 20 / 1 volume ratio), obtained 0.48mmol purple solid compound III-2, yield: 96%. (The yield of compound III-2 is the molar amount of compound III-2 / the molar amount of osmalpentyne compound IV-5×100...
Embodiment 3
[0142]
[0143] [Os] in the above formula is OsCl(PPh 3 ) 2 . Among them, HCl·Et 2 O is diethyl ether hydrochloride (purchased from Sarn Chemical Technology (Shanghai) Co., Ltd., the brand name is A01W8101821000), (p-thiomethylphenylacetylene, reference RSC Advances, 6(95), 92845-92851, 2016 synthesis), the DCM used is dichloromethane redistilled solvent.
[0144] Preparation of Compound III-3
[0145] Under nitrogen atmosphere, under magnetic stirring, dissolve osmalpentyne compound I-88 (0.5mmol) and p-thiomethylphenylacetylene II-5 (0.5mmol) in 50mL of dichloromethane, and quickly add 2mol 3mL of diethyl ether hydrochloride solvent per L, reacted at room temperature for 2h, concentrated, neutral alumina column chromatography (eluent: dichloromethane / methanol=20 / 1 volume ratio), and obtained 0.49mmol purple solid compound III-1 , Yield: 98%. (The yield of Compound III-3 is the molar amount of Compound III-3 / the molar amount of Ospentyne Compound I-88×100%).
[014...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com