Monosubstituted metal phthalocyanine and synthesis and separation method and application thereof
A metal phthalocyanine and separation method technology, applied in the direction of medical preparations containing active ingredients, organic active ingredients, drug combinations, etc., can solve the problems of limited experimental conditions, unsuitable for wide application, etc., achieve low phototoxicity and side effects, Enhanced selective uptake rate, clear composition and structure effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
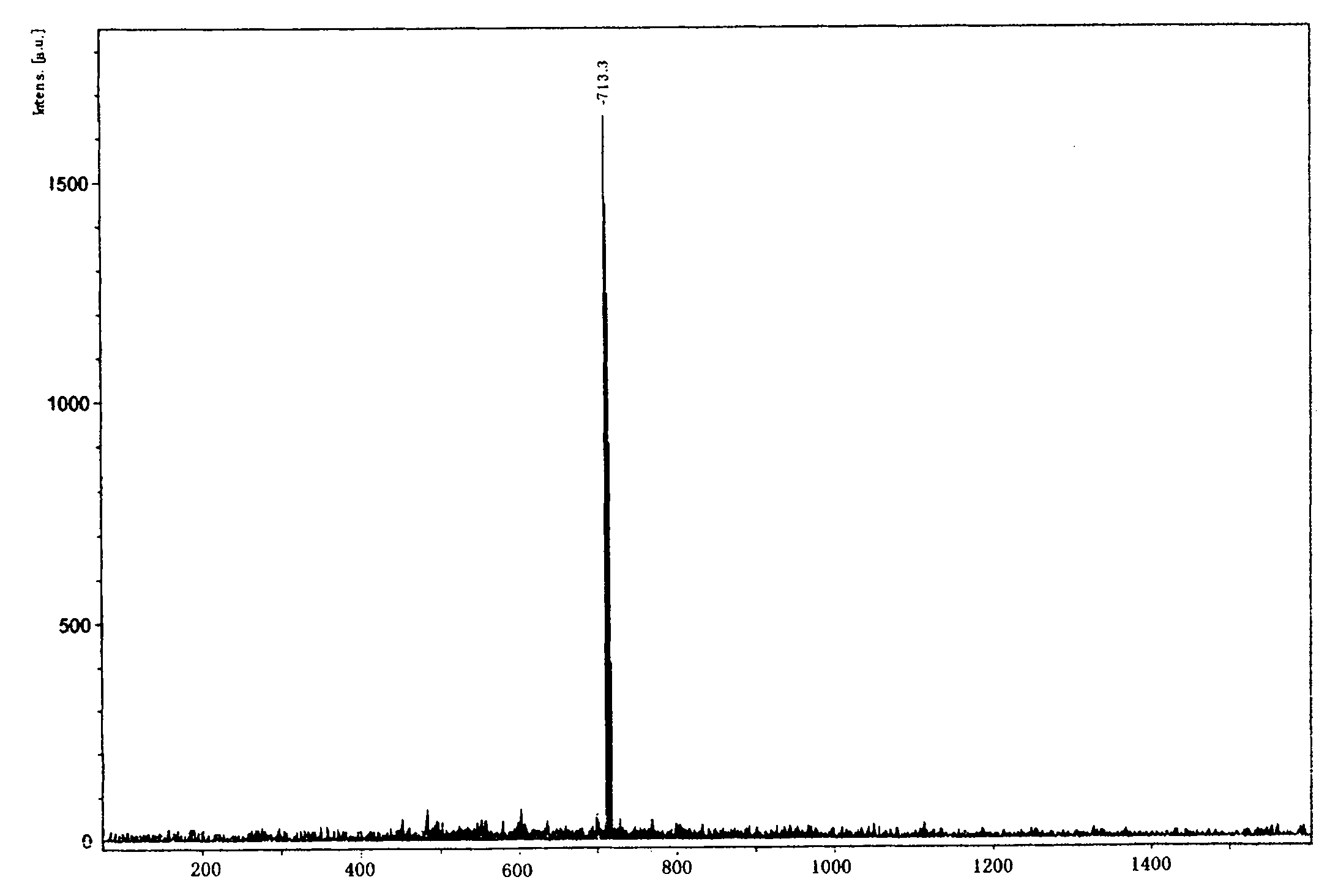
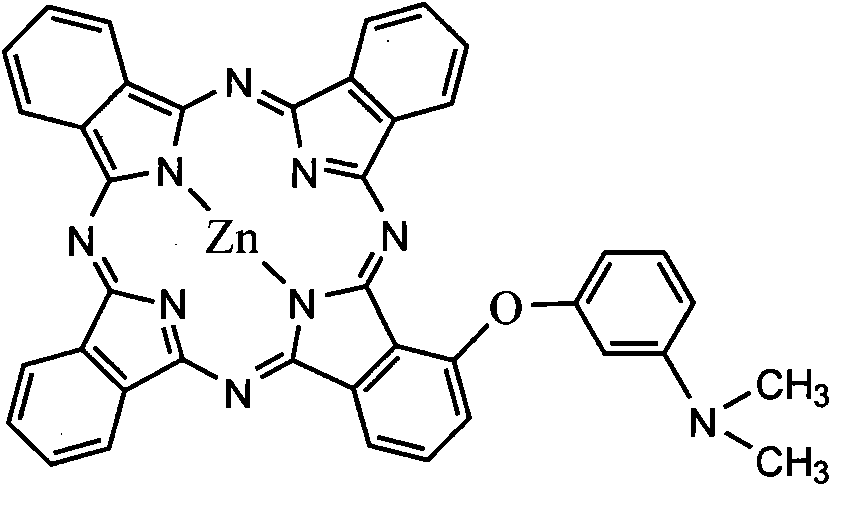
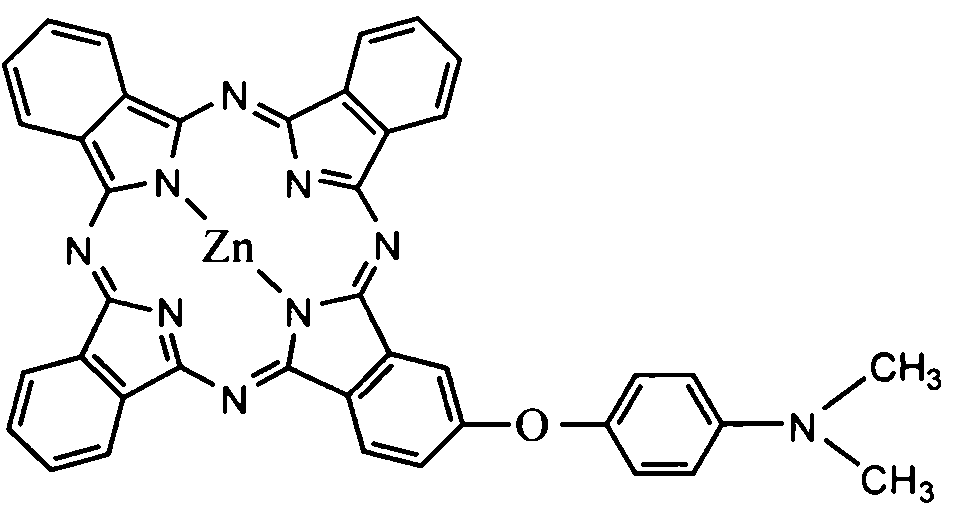
[0033] Mono-substituted metal phthalocyanine, the structural formula of the mono-substituted metal phthalocyanine is:
[0034] Me in the described structural formula represents a divalent metal atom, and taking zinc Zn as an example, R in the structural formula is C 1 -C 10 substituted alkyl with tertiary nitrogen or C 1 -C 10 The substituted aralkyl group with tertiary nitrogen, taking (dimethylaminophenoxy group) as an example, according to the molar ratio, the structural formula is The substituted phthalonitrile 1 and phthalonitrile 3 are reacted in n-pentanol, the molar ratio of the n-pentanol to the substituted phthalonitrile is 10:1, and the reaction is preferably at 130 °C, the reaction time is preferably 22 hours, and a mixture containing mono-(dimethylaminophenoxy) substituted metal phthalocyanine is generated, and the obtained mixture is separated by dry column chromatography.
Embodiment 2
[0036] In the above synthesis and separation method of monosubstituted metal phthalocyanine, the metal salt is acetate or metal halide, taking zinc acetate as an example, the molar ratio of zinc acetate to substituted phthalonitrile is preferably 1:1 ; The organic base is 1,8-diazabicyclo[5,4,0]undecene-7 (DBU) or 1,5-diazabicyclo[4,3,0]nonane- 5-ene (DBN) or pyridine, preferably DBU, the molar ratio of DBU to substituted phthalonitrile is preferably 4:1; the reaction is preferably at 120°C-200°C, especially at 130°C, and the reaction time is preferably 22 Hour.
Embodiment 3
[0038] The above monosubstituted metal phthalocyanine and its synthesis and separation method, the dry column chromatography comprises the following steps: a. dissolving the mixture and loading it on a separation column containing dry silica gel; b. The mixture is separated by washing the separation column with an eluent containing tetrahydrofuran and / or n-hexane and / or petroleum ether.
[0039] The separation of the present invention uses dry column chromatography for chromatographic separation, using a standard glass chromatography column and commercially available silica, and using the above-mentioned eluent to rinse, so that the nitrogen-containing monosubstituted phthalocyanine complex can be obtained Good separation. Dry column chromatography is an improved chromatographic technique. All mixtures that can be separated by thin layer chromatography, including those that cannot be separated on the "wet column", can be separated on the dry column. Using this method, prepara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com