Synthesis of cyanine, coumarin and dicarbonyl boron fluoride hybrid fluorochrome and application thereof
A technology of boron dicarbonyl fluoride and coumarin, which is applied in the field of synthesis of new hybrid fluorescent dyes, can solve problems such as limited application range, small Stokes shift, and short absorption and emission wavelengths, and achieves short synthesis routes and optical Improved properties and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of intermediate 1: Weigh 5.80g (0.03mol) of 4-diethylamino salicylaldehyde in a 250mL flask and 3.78g (0.03mol) of 4-hydroxy-6-methylpyrone dissolved in 50mL of water, then add 0.345 g (0.003mol) L-proline, stirred and refluxed at 80°C for 2 hours, a large amount of brown solid precipitated out, and the reaction was detected by TLC until the reaction was complete. After cooling, the brown solid was filtered out with suction, recrystallized with 40 mL of ethanol, and vacuum-dried after suction filtration to obtain 7.86 g of yellow-brown crystals with a yield of 86.9%. 1 H NMR (500MHz, CDCl 3 )δ16.26(s,1H),8.52(s,1H),7.40(d,J=8.8Hz,1H),6.99(d,J=1.9Hz,1H),6.63(dd,J=9.0,2.4 Hz, 1H), 6.49(d, J=2.3Hz, 1H), 3.47(q, J=7.1Hz, 4H), 2.22(s, 3H), 1.25(t, J=7.1Hz, 6H).
[0025] Weigh 3.01g (0.01mol) of yellow-brown crystals in a 100mL dry flask, dissolve in 20mL of dichloromethane, and add 4.26g (0.03mol) of boron trifluoride ether. Under the protection of nitrogen, t...
Embodiment 2
[0027] Preparation Ia:
[0028]
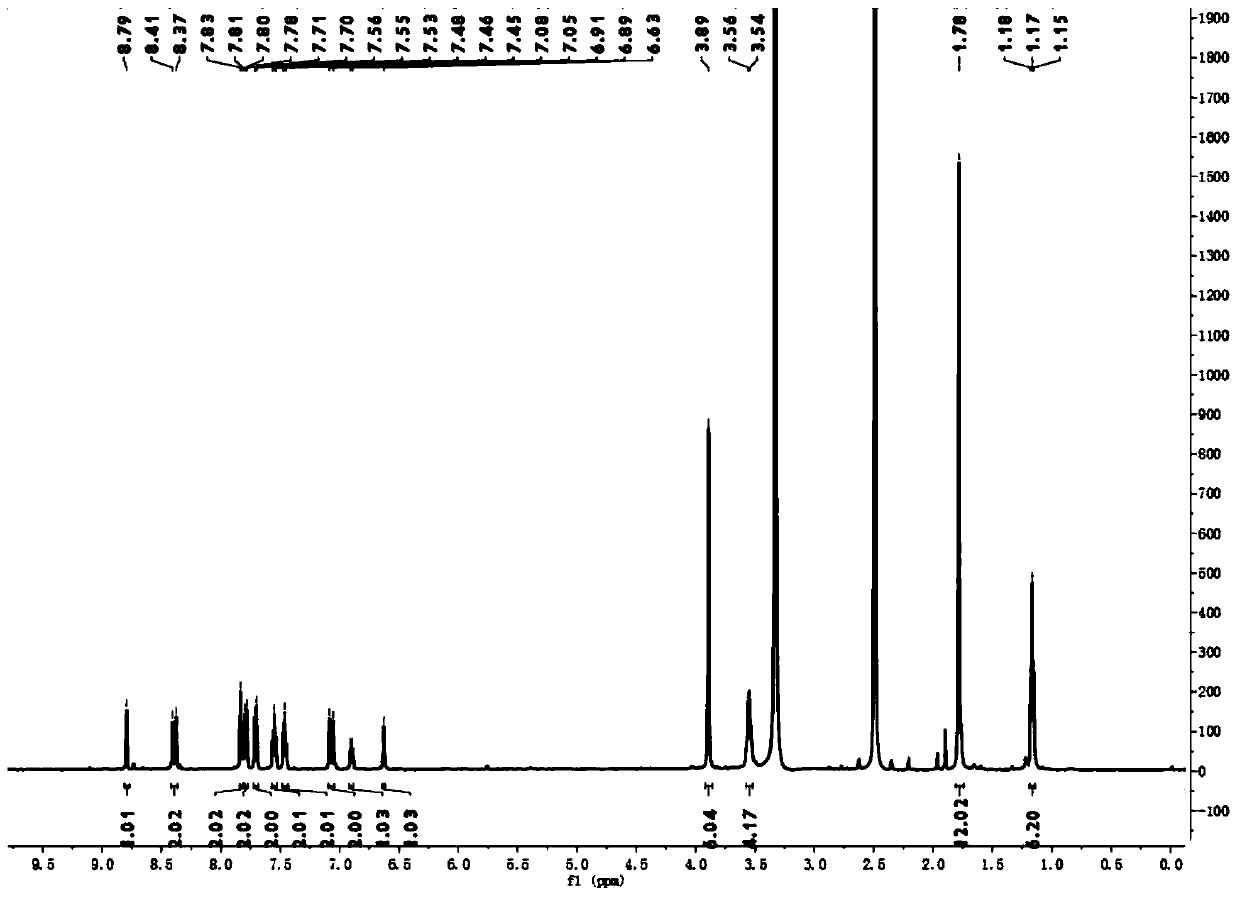
[0029] Weigh 0.35g (1mmol) of intermediate 1 in a 50mL flask, dissolve it in 10mL of acetic anhydride, add 0.402g (2mmol) of 1,3,3-trimethyl-2-methylene indoline acetaldehyde, at 60°C Reflux and stir for 3 h, TLC detects the reaction until the reaction is complete. After adding ice water, a solid precipitated out. After suction filtration, it was washed with perchloric acid and 5 mL of ethanol, and dried in vacuo to obtain 0.38 g of a dark blue solid with a yield of 57.5%. 1 H NMR (500MHz, DMSO) δ8.79(s, 1H), 8.39(d, J=15.6Hz, 2H), 7.82(d, J=10.3Hz, 2H), 7.79(d, J=7.4Hz, 2H ),7.71(d,J=7.9Hz,2H),7.55(t,J=7.7Hz,2H),7.46(t,J=7.4Hz,2H),7.07(d,J=15.6Hz,2H), 6.90(d,J=9.2Hz,1H),6.63(s,1H),3.89(s,6H),3.55(d,J=6.9Hz,4H),1.78(s,12H),1.17(t,J =6.9Hz,6H).
Embodiment 3
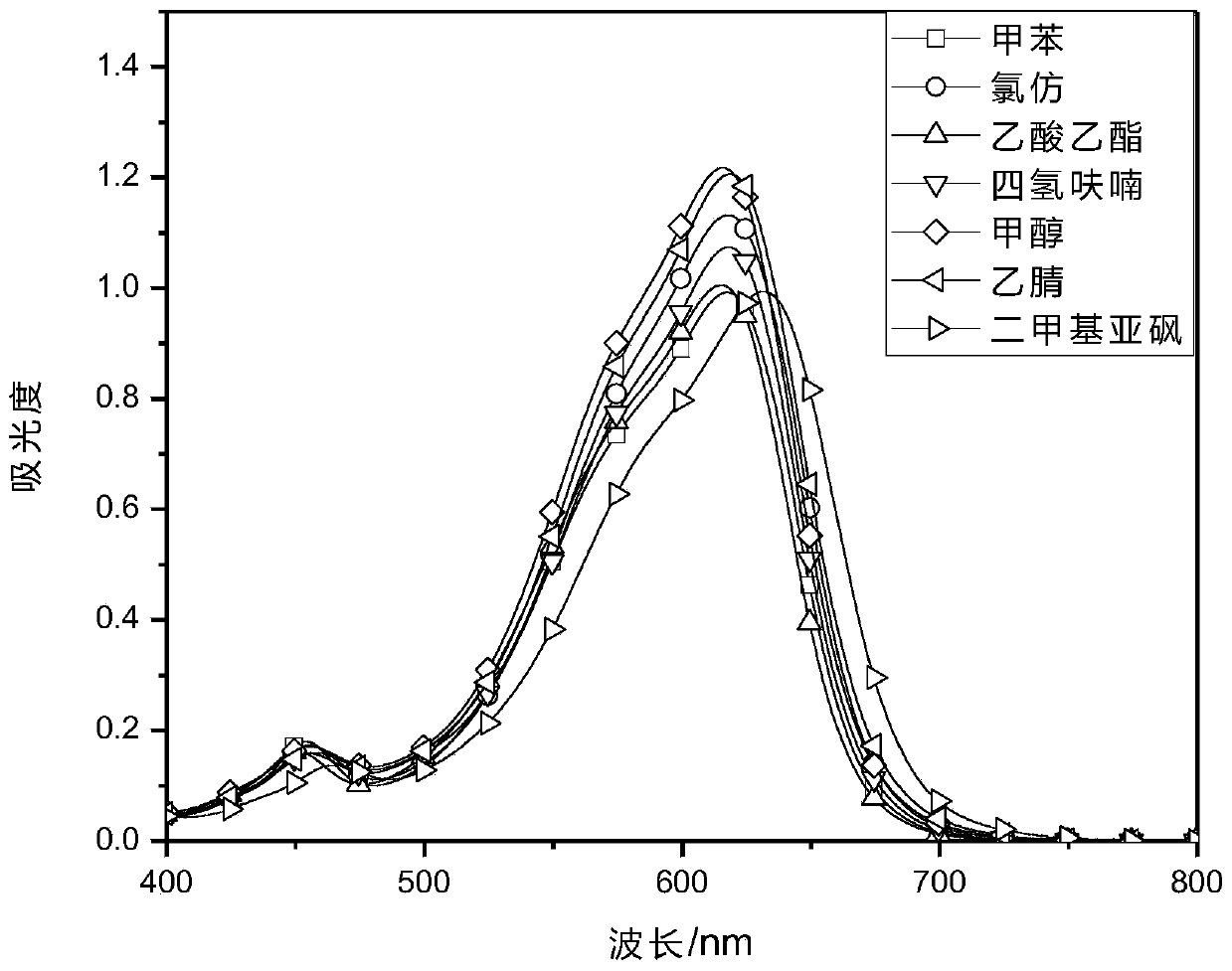
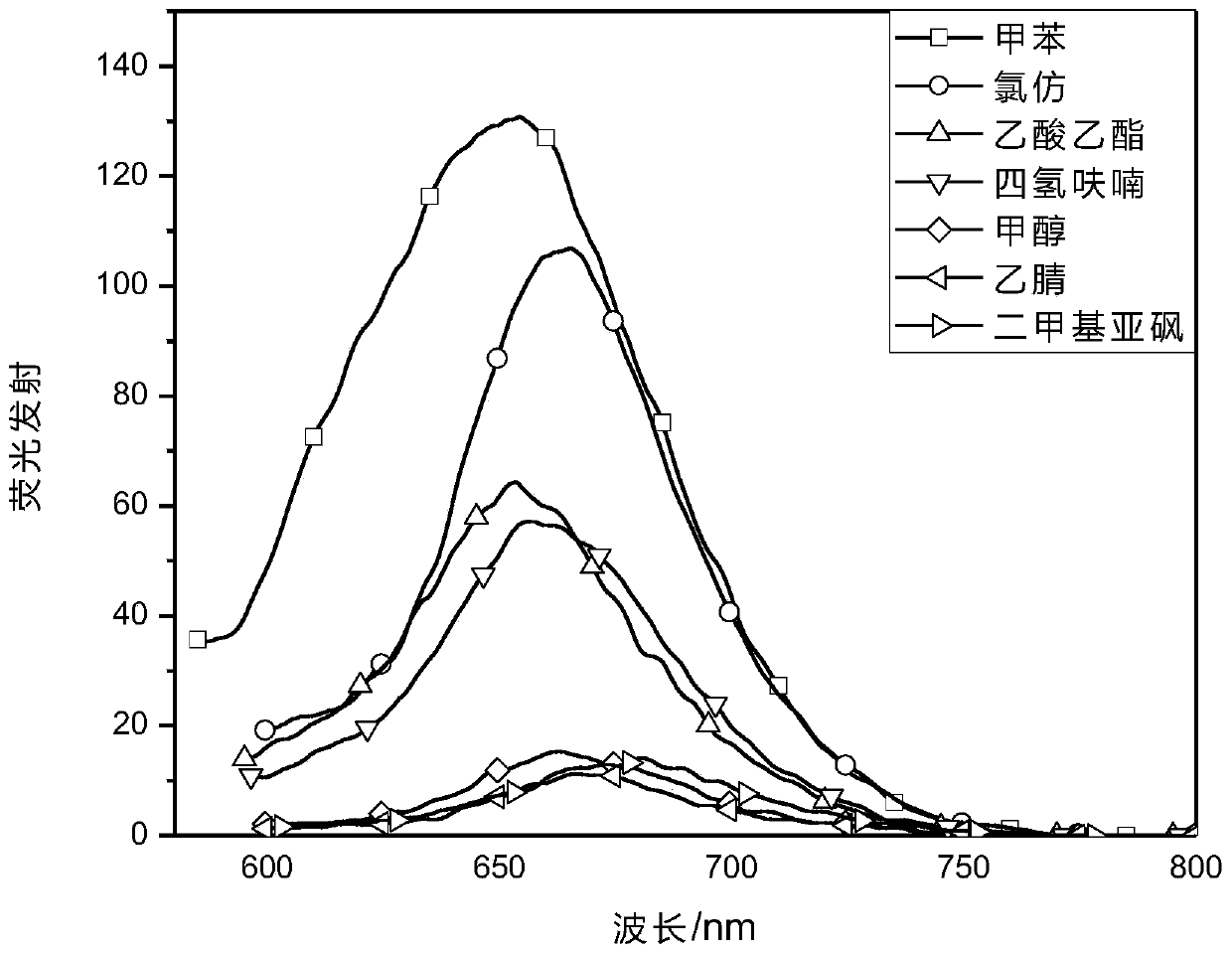
[0031] Prepare 10μmol / L Ia in different solvents, and test its ultraviolet-visible absorption spectrum and fluorescence emission spectrum.
[0032] The photophysical properties of the target molecule I in THF in the above examples are shown in Table 1:
[0033] Table 1: Photophysical properties of Ⅰ
[0034]
[0035] The results in Table 1 show that the maximum absorption wavelength of dye I in tetrahydrofuran is 617nm, and the maximum emission wavelength is 657nm. The strong molar absorptivity indicates the application prospect of compound I in the direction of luminescent materials, organic dyes and organic probes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com