2-methyl-6-tert-butyl-4-dicyanmethylene-4H-pyran synthesis method
A technology of dicyanomethyl group and synthesis method, applied in directions such as organic chemistry, can solve the problems of high cost, reduced reaction yield, low yield and the like, and achieves the effects of mild reaction conditions, easy production and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、5
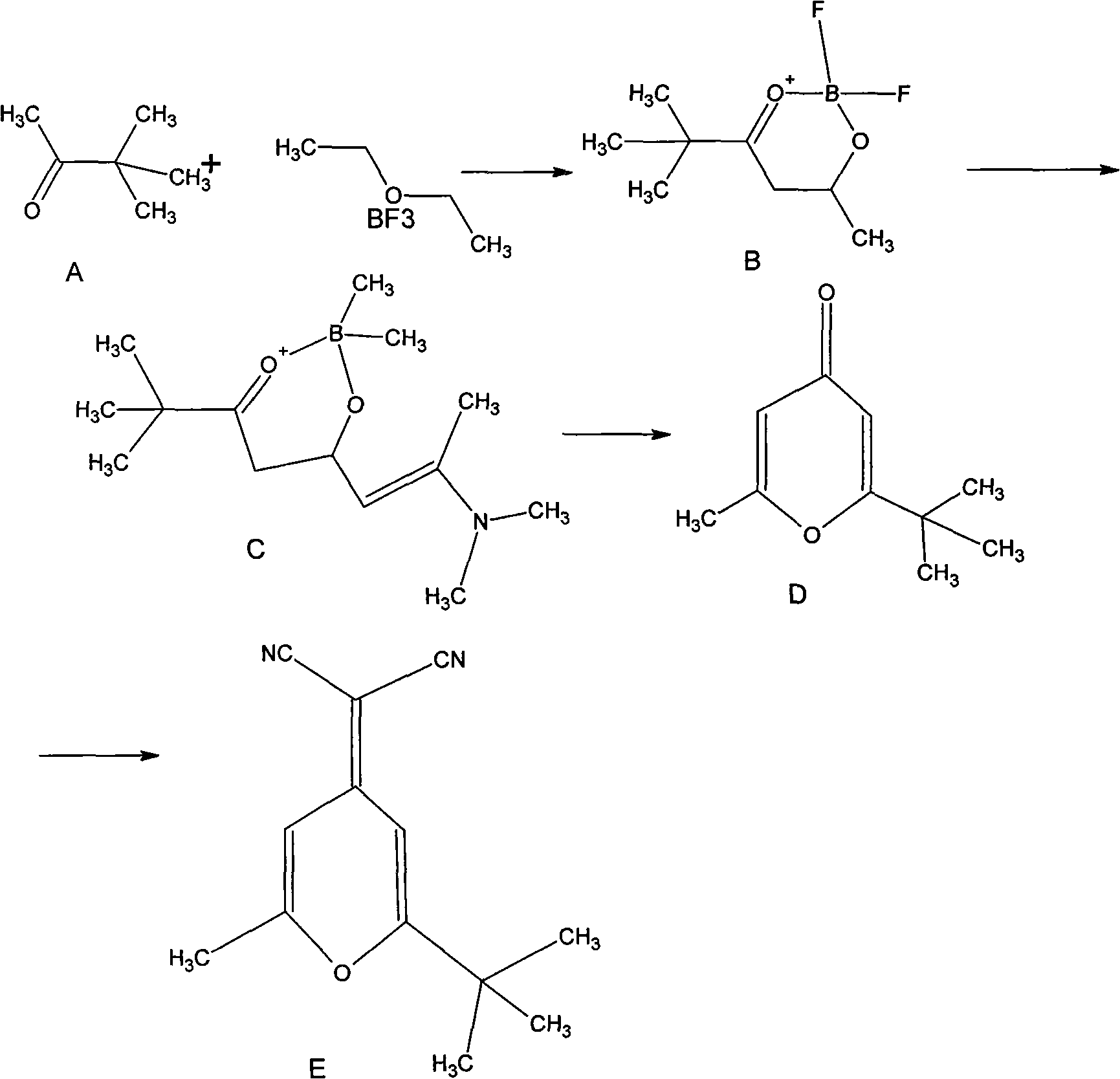
[0038] Example 1, 5,5-dimethyl-2,4-hexanedialdehyde-O, O-boron difluoride
[0039] Place a 3000ml three-necked flask in an ice-salt bath, under the protection of nitrogen, add 100g of pinacolone and 204g of acetic anhydride, mix and stir, cool with ice-salt, then add 500g of boron trifluoride etherate complex dropwise, after the addition is complete The mixture was reacted overnight at room temperature. Under ice-salt cooling, 10% sodium hydroxide was added dropwise until PH=7, static, liquid separation, and the organic phase was concentrated to precipitate 120 g of a light yellow solid with a yield of 60% (US5935720 reported a yield of 20%).
[0040] Melting point: 82~84℃
[0041] HNMR (CDCl 3 , 400Hz): 1.19(9H, s); 2.2487(3H, s); 5.97(1H, s)
Embodiment 2
[0042] Example 2, 7-dimethylamino-2,2-dimethyl-6-ene-3,5-octanedial-O,O-boron difluoride
[0043] In a 2000ml three-necked flask, under nitrogen protection, add 275g of compound B, 245g of N,N-dimethylacetamide, and 14ml of 2,6-lutidine, and stir. Heated to 30°C, added dropwise N,N-dimethylacetamide dimethyl acetal 388g, after the dropwise addition, kept the temperature and continued to stir for 3 hours, then raised the temperature to 85°C, TLC detected that the reaction of the raw materials was complete, and stopped the reaction (10 hours ), the mixture was a reddish-brown oily liquid, the reaction solution was cooled overnight at -10°C to 0°C, filtered, and the product was washed with petroleum ether to obtain 240 g (85%) of a pale yellow solid. (US5935720 reports 36% yield)
[0044] Melting point: Decompose above 220°C
[0045] HNMR (CDCl 3 , 400Hz): 1.29(9H, s); 2.62(3H, s): 3.19(6H, s); 4.88(1H, s); 5.47(1H, s)
Embodiment 3
[0046] Embodiment 3, 2-methyl-6-tert-butyl-pyrone
[0047] In a 5000ml three-necked flask, add 615g of reaction product C, then add 1080g of water and 1080ml of ethanol in turn, cool with ice water, add 1080ml of 70% perchloric acid, heat and reflux overnight, TLC detects that the reaction of raw materials is complete, stop heating, cool, and rotate to evaporate Spin to dry ethanol, add ammonia water to adjust to alkalinity, a solid precipitates, filter the solid to obtain 300 g (76%) of a yellow product. (US5935720 reports yield 75%)
[0048] HNMR (DMSO, 400Hz): 1.23 (9H, s); 2.25 (3H, s); 6.04 (1H, m); 6.12 (1H, m)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com