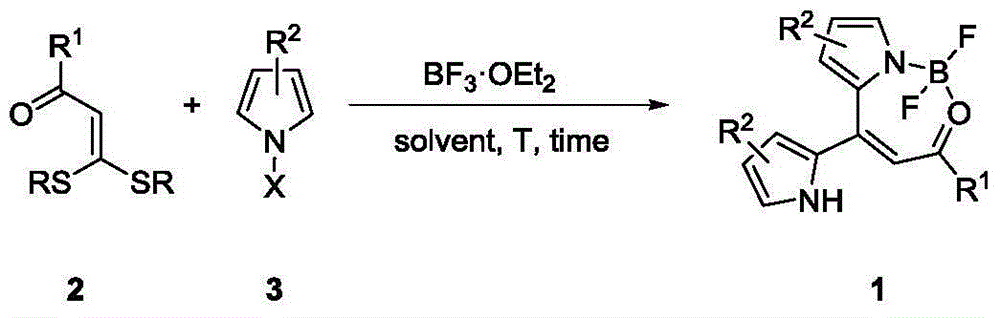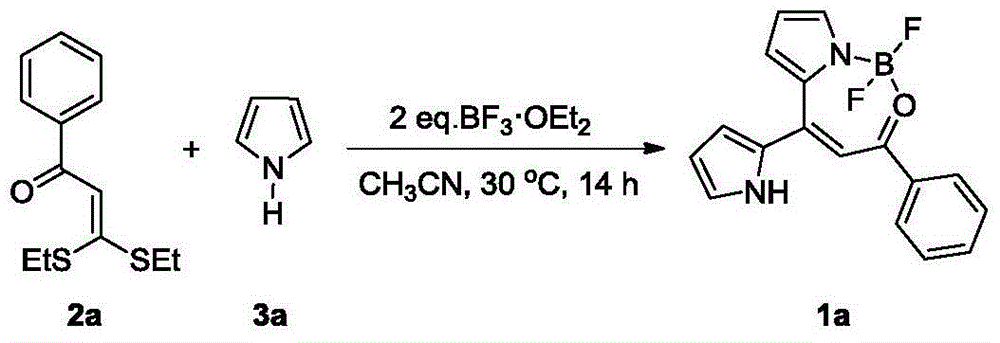Synthesis method of dipyrromethene N, O-boron difluoride derivative
A dipyrromethene and boron difluoride technology, which is applied in the field of synthesis of dipyrromethene N,O-boron difluoride derivatives, can solve the problems of complex preparation process, many reaction steps, and complex process , to achieve the effect of simple preparation, simple steps, and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] To a 10 mL Schlenk reaction flask, add 2a (126.2 mg, 0.5 mmol), 5 mL of acetonitrile, N-H pyrrole 3a (67.1 mg, 1.0 mmol) and BF 3 ·OEt 2 (141.9 mg, 1.0 mmol). The reaction was stirred at 30 °C for 14 h. TLC showed that after the reaction was over, add 5mL saturated NaHCO 3 Aqueous solution was used to quench the reaction, the reaction solution was transferred to a separatory funnel, water (30 mL) was added, extracted with dichloromethane (3×10 mL), and the organic phases were combined. Dry over anhydrous magnesium sulfate and filter. The volatile components were removed, and then separated by silica gel column chromatography (eluent was petroleum ether (60-90°C / ethyl acetate, v / v=3:1) to obtain the target product 1a (100.8mg, yield 65% ).
Embodiment 2
[0030] The reaction steps and operations are the same as in Example 1, except that the solvent is dichloromethane. The reaction was stopped, and the target product 1a (46.5 mg, yield 30%) was obtained after post-processing. It shows that dichloromethane with low polarity is not as effective as acetonitrile with high polarity as a solvent.
Embodiment 3
[0032] The reaction steps and operations are the same as in Example 1, except that the reaction solvent is nitromethane. The reaction was stopped, and the target product 1a (96.1 mg, yield 62%) was obtained after post-processing. It shows that the effects of nitromethane and acetonitrile are roughly the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com