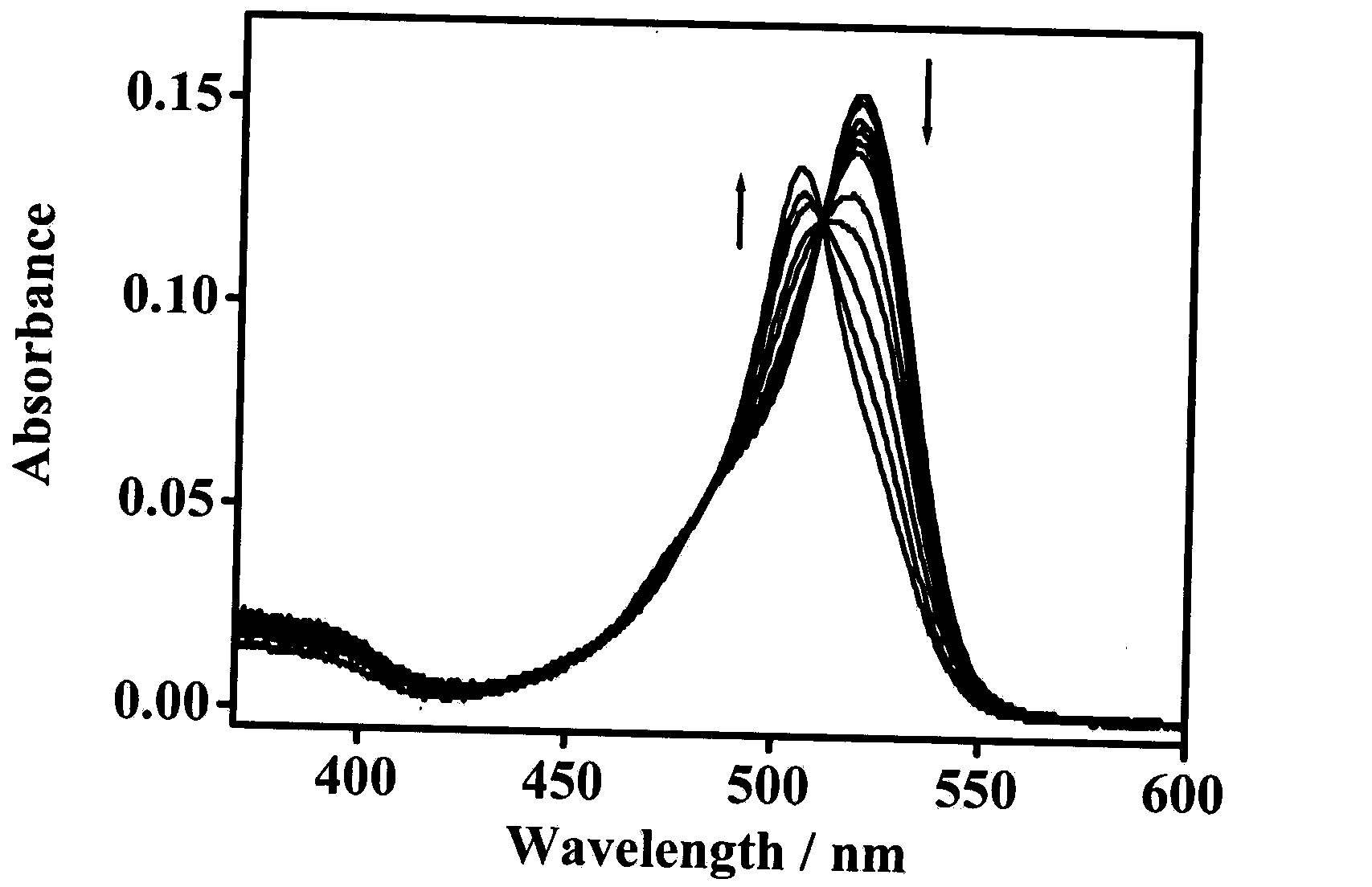
Synthesis and application of fluorescence molecular probes for detecting mercaptoamino-acids by virtue of naked eyes and fluorescence enhancement
A technology of fluorescent molecular probes and thiol amino acids, which is applied in the field of chemical analysis and detection, can solve problems such as changes in fluorescence intensity, and achieve the effects of wide detection range, low detection limit, and stable optical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Preparation of boron difluoride-dipyrromethane derivatives
[0029] Under the protection of argon, dissolve 2,4-dimethylpyrrole (0.95g, 10mmol) and benzaldehyde (0.53g.5mmol) in 200mL of dichloromethane, add 1 drop of trifluoroacetic acid as catalyst, and stir at room temperature for 6 Hour. Dissolve 0.9g DDQ in 75mL dichloromethane and add to the above reaction system, then add 5ml DIEPA and 10mL boron trifluoride ether into the reaction system, continue stirring for 1 hour, pour the reaction solution into 100mL water to quench reaction. Extracted with dichloromethane, dried over anhydrous sodium sulfate, and separated by column chromatography (petroleum ether / dichloromethane=1:1) to obtain 0.22 g of an orange-red solid product (yield: 45%), namely boron difluoride- Dipyrromethane derivatives.
[0030] 1 HNMR (300MHz, CDCl 3 ):δ ppm =7.48(3H, d, J5.1), 7.28(2H, s), 5.98(2H, s), 2.56(6H, s), 1.37(6H, s).
Embodiment 2
[0031] Example 2: Preparation of boron difluoride-dipyrromethaldehyde derivatives
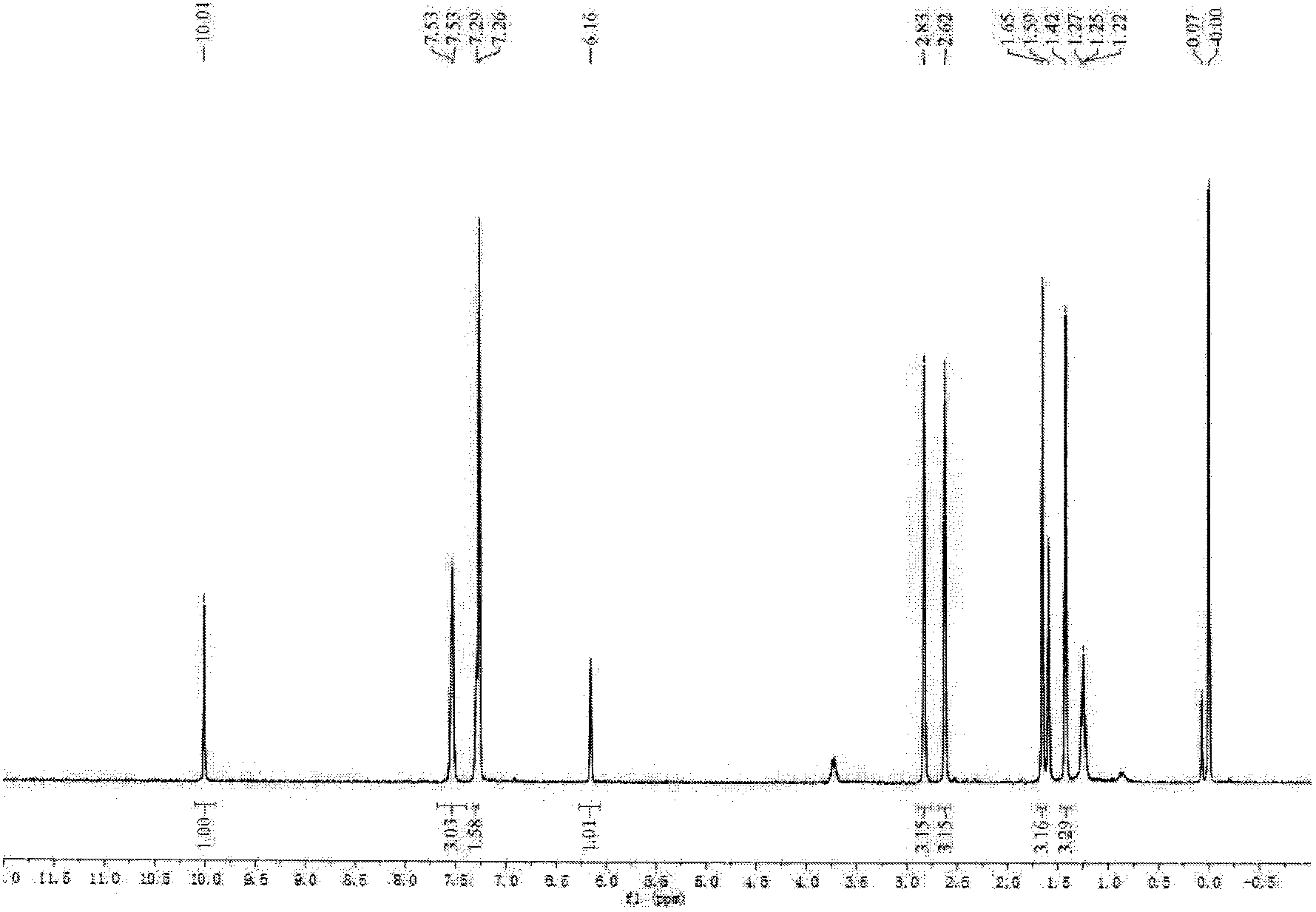
[0032] Under argon protection, mix 6mL DMF and 6mL phosphorus oxychloride in an ice bath, return to room temperature after 5 minutes, continue stirring for 30 minutes, add boron difluoride-dipyrrole dissolved in 60mL dropwise to the reaction solution Methane derivative (162mg, 0.5mmol) in 1,2-dichloroethane was heated to 50°C and stirred for 2 hours. After the reaction was completed, it was lowered to room temperature, poured into 100 mL of saturated sodium bicarbonate solution for neutralization, and continued to stir for 30 minutes. It was then extracted with dichloromethane, dried over anhydrous sodium sulfate, and separated by column chromatography (petroleum ether / ethyl acetate=8:1) to obtain 70 mg of the product (yield: 40%) which was boron difluoride-dipyrromethaldehyde derivative.
[0033] 1 HNMR (300MHz, CDCl 3 ):δ ppm =10.01(1H, s), 7.53(3H, d, J2.5), 7.29(2H, s), 6.16(1H, s), 2....
Embodiment 3
[0034] Embodiment 3: Preparation of molecular fluorescent probe
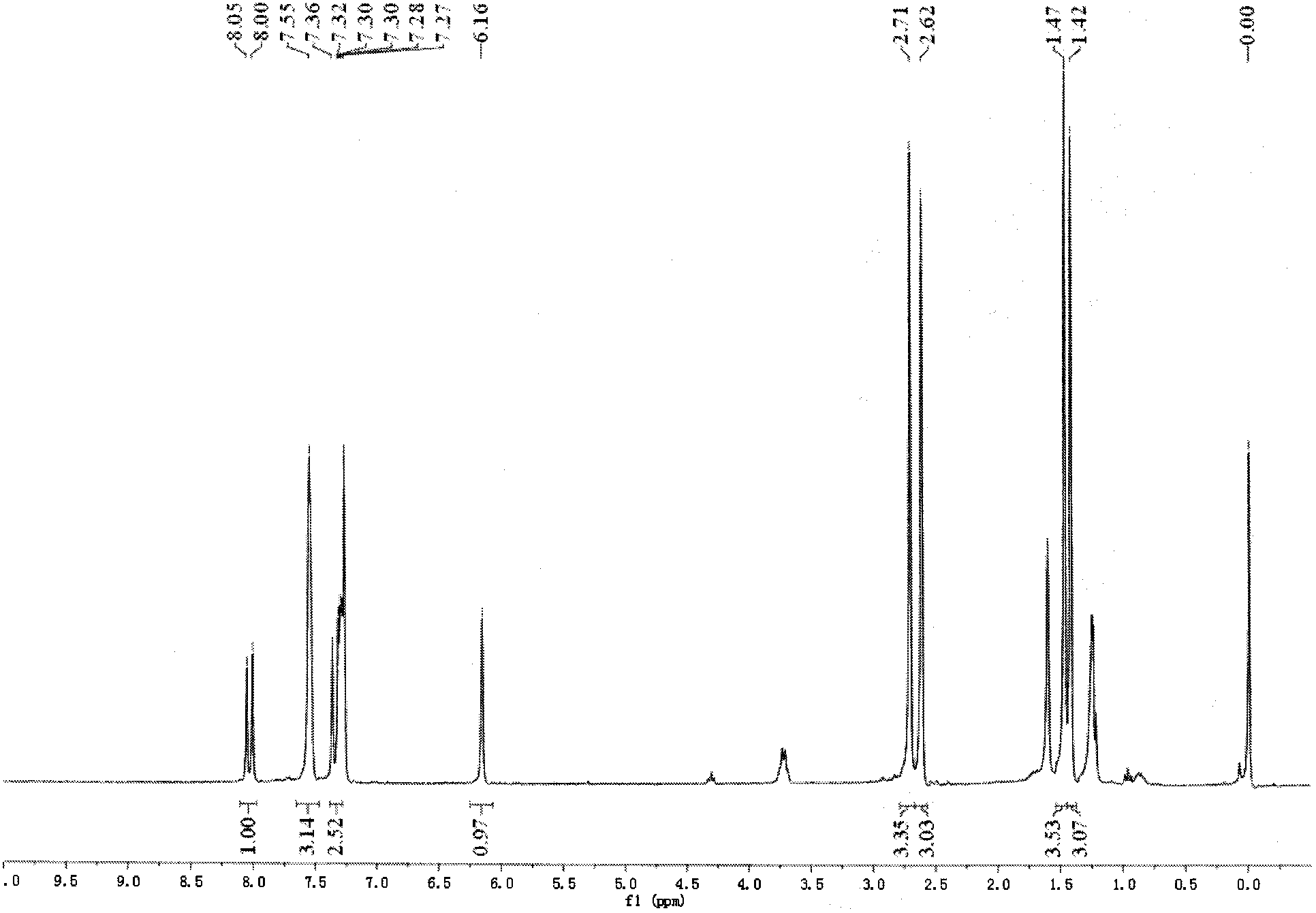
[0035] Under argon protection, dissolve boron difluoride-dipyrromethaldehyde derivatives (70mg, 0.2mmol) and nitromethane (50mg, 0.8mmol) in 10mL toluene, add 1 drop of piperidine and 1 drop of acetic acid, and reflux 2 hours. After cooling down, it was extracted with dichloromethane, dried over anhydrous sodium sulfate, and separated by column chromatography (petroleum ether / dichloromethane=1:1) to obtain 53 mg of the product (yield: 68%), which was the probe compound.
[0036] 1 HNMR (300MHz, CDCl 3 ):δ ppm =8.03(1H, d, J13.7), 7.55(3H, s), 7.39-7.27(3H, m), 6.16(1H, s), 2.71(3H, s), 2.62(3H, s), 1.47 (3H, s), 1.42 (3H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com