Triphenylamine two-photon fluorescence probe compound and preparation method and application thereof
A two-photon fluorescence and triphenylamine technology, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve problems such as harming human health, increasing environmental pollution, and calmness, and achieves non-destructive detection, high reactivity, and The effect of improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
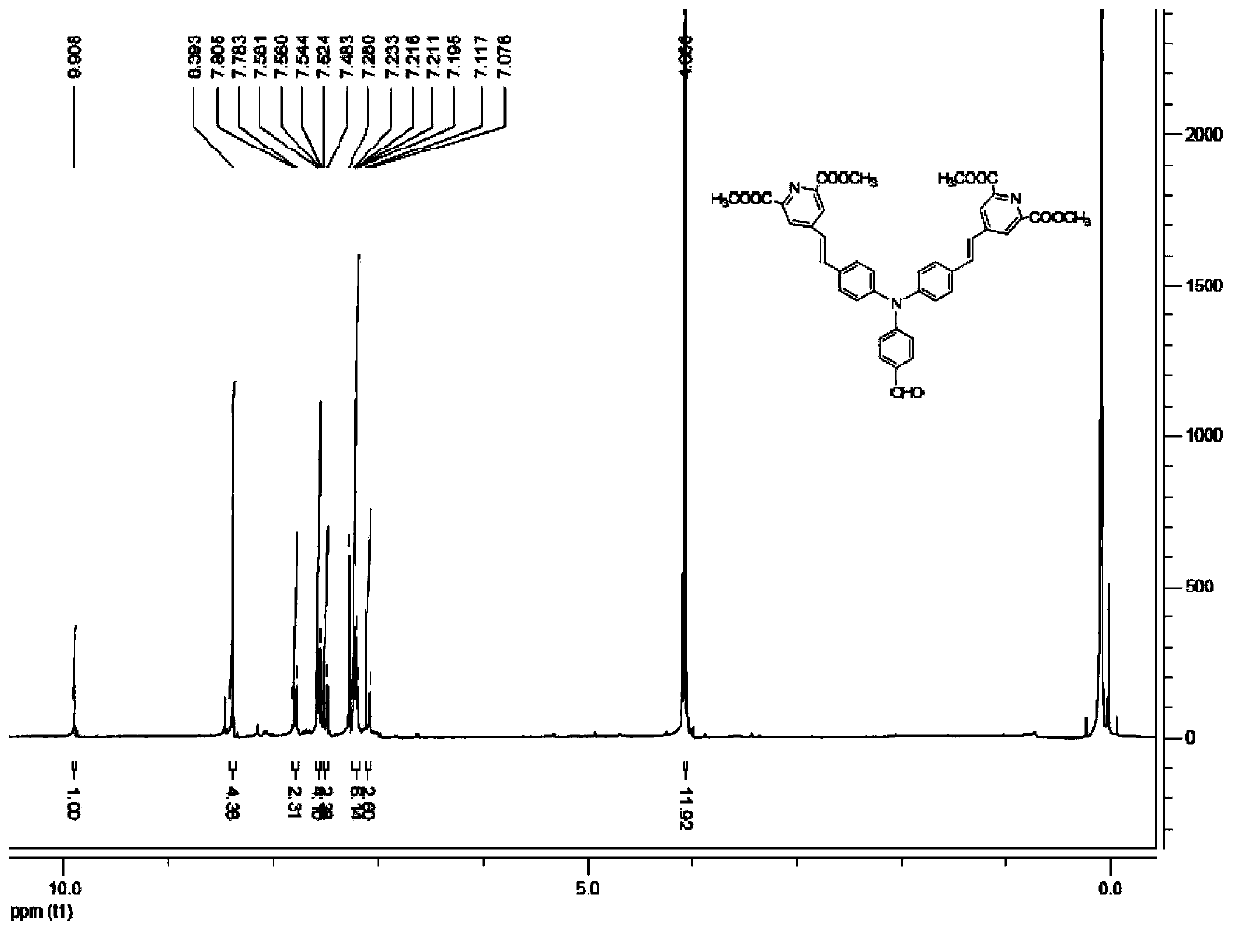
[0043] 1. Synthesis of dimethyl chloride-4-methylenetriphenylphosphino-pyridine-2,6-dicarboxylate
[0044] (1) Synthesis of pyridine-2,6-dicarboxylic acid
[0045]Put 500mL of distilled water and 12mL (92mmol) of 2,6-lutidine in a 1000mL round-bottomed flask, slowly add 80g (506mmol) of potassium permanganate under magnetic stirring, and insert a reflux condenser. Reflux at 110°C-115°C for 3.5h. The reaction was stopped, cooled to room temperature, and the black solid was removed by suction filtration. The colorless filtrate was collected, and four fifths of the filtrate was removed under reduced pressure. With continuous stirring, slowly pour about 20 mL of sulfuric acid with a volume concentration of 70%, and a large amount of white crude product is precipitated. After cooling, filter with suction to obtain pyridine-2,6-dicarboxylic acid.
[0046] (2) Synthesis of dimethyl pyridine-2,6-dicarboxylate
[0047] Put 33.5g (200mmol) of pyridine-2,6-dicarboxylic acid, 120mL (1...
Embodiment 2
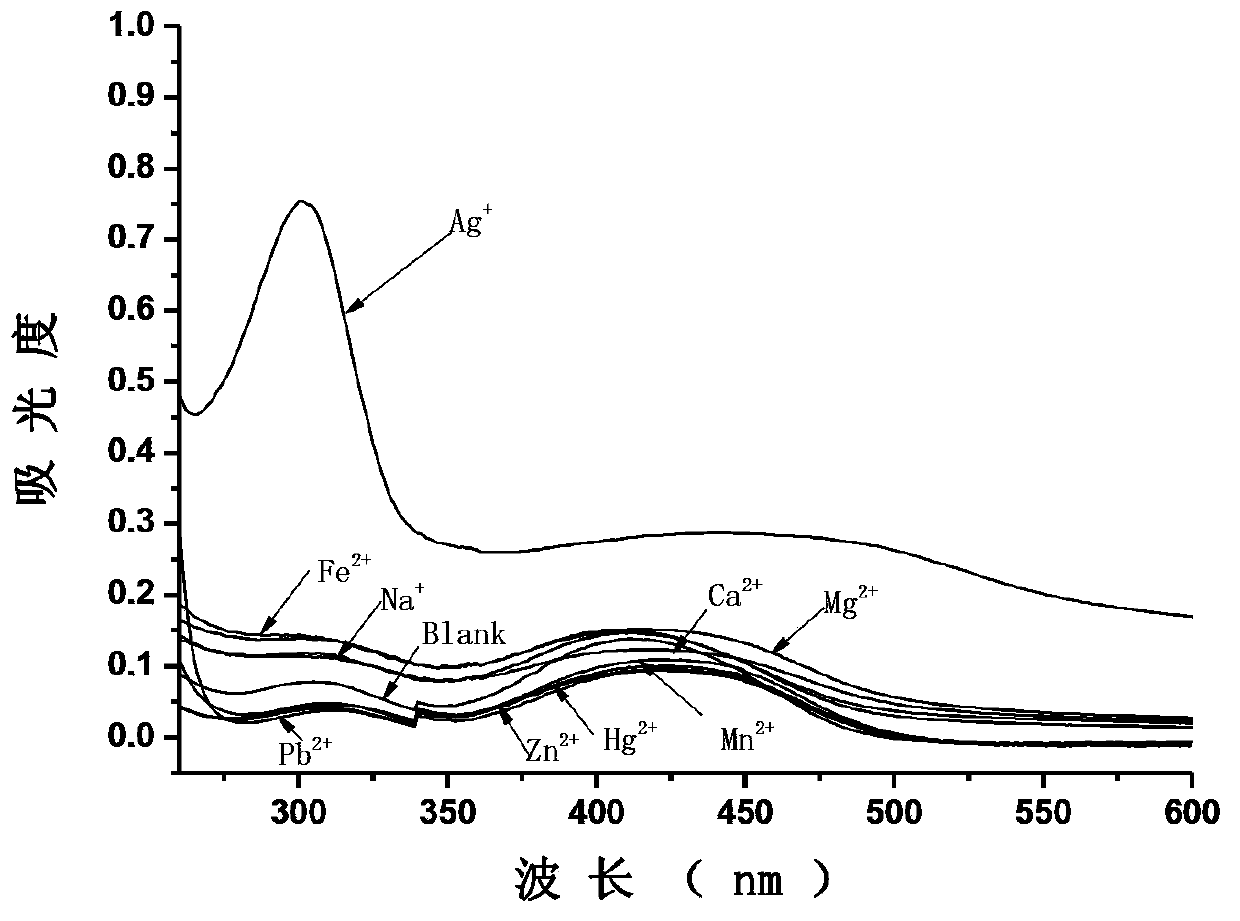
[0061] The selectivity of compound I to silver ion
[0062] Using the probe compound I synthesized in Example 1, the selectivity to silver ions was evaluated by absorption spectroscopy. Dissolve compound I in a mixed solvent of dimethyl sulfoxide: distilled water = 5:95 (volume ratio), and prepare compound I with a concentration of 1×10 -5 mol / L mixed solution, add different metal ions respectively, so that the concentration of metal ions is 1×10 -5 mol / L, test its single-photon absorption spectrum, the specific results are as follows image 3 shown, from image 3 It can be seen that compound I has high selectivity to silver ions (the metal ion is Ag + 、Na + , Ca 2+ 、Cd 2+ , Fe 2+ , Zn 2+ , Hg 2+ , Mg 2+ , Pb 2+ , Mn 2+ ), when silver ions were added to the solution of compound I, the absorbance of the solution around 305nm increased significantly; while other metal ions were added to the solution of compound I, the absorbance hardly changed.
[0063] It is 1×10 t...
Embodiment 3
[0065] Absorption responses of compound I to different concentrations of silver ions
[0066] Using the probe compound I synthesized in Example 1, dissolve compound I in a mixed solvent of dimethyl sulfoxide:distilled water=5:95 (volume ratio), and prepare the compound I with a concentration of 1×10 -5 mol / L mixed solution, adding different concentrations of silver ions, the concentration of silver ions changes from small to large in order of 0, 1×10 -7 mol / L, 5×10 -7 mol / L, 1×10 -6 mol / L, 2×10 -6 mol / L, 4×10 -6 mol / L, 6×10 -6 mol / L, 8×10 -6 mol / L, 1×10 -5 mol / L, 2×10 -5 mol / L, 4×10 -5 mol / L, 6×10 -5 mol / L, 8×10 -5 mol / L and 1×10 -4 mol / L, specifically as Figure 5 As can be seen from the figure, the single-photon absorbance of Compound I of the present invention is positively related to the concentration of silver ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com